FERINJECT®
(ferric carboxymaltose)
Find out more about how treatment of iron deficiency with Ferinject® (ferric carboxymaltose) could support your treatment goals for patients
Prescribing information
FERINJECT®
(ferric carboxymaltose)
Find out more about how treatment of iron deficiency with Ferinject® (ferric carboxymaltose) could support your treatment goals for patients
Prescribing informationFerinject is indicated for the treatment of iron deficiency when: oral iron preparations are ineffective; oral iron preparations cannot be used; or there is a clinical need to deliver iron rapidly. The diagnosis of iron deficiency must be based on laboratory tests.1
- Ferinject is approved for adults and paediatric patients aged 1 year and older1
Guidelines
Guideline recommendations for the use of intravenous (IV) iron in patients with iron deficiency (ID)2–7
Key learning points:
- IV iron is recommended by multiple guidelines for the treatment of ID or iron deficiency anaemia (IDA) in patients with heart failure (HF), chronic kidney disease (CKD), pre-operative anaemia or gastrointestinal (GI) bleeding2–7
- The European Society of Cardiology (ESC) recommends IV iron supplementation in symptomatic patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with mildly-reduced ejection fraction (HFmrEF) and ID,* to alleviate HF symptoms and improve quality of life2,3
*Defined as serum ferritin <100 ng/mL or serum ferritin 100-299 ng/mL with TSAT<20%.2
Explore guidelines for IV iron in different patient groups:
ESC guidelines (2021 and 2023 focused update) for the diagnosis and treatment of acute and chronic HF2,3

Recommendation for regular screening of iron deficiency remains unchanged:
- ESC 2021 guidelines recommend that all patients with HF be periodically screened for anaemia and ID with a full blood count, serum ferritin concentration, and TSAT (Class I, level C)2
Recommendations for the treatment of ID from the ESC 2023 focused update:
- IV iron supplementation is recommended in symptomatic patients with HFrEF and HFmrEF, and ID, to alleviate HF symptoms and improve quality of life (Class I, Level A)3
- IV iron supplementation with ferric carboxymaltose or ferric derisomaltose should be considered in symptomatic patients with HFrEF and HFmrEF, and ID, to reduce the risk of HF hospitalisation (Class IIa, Level A)3
ID is defined as serum ferritin of <100 mcg/L or serum ferritin of 100-299 mcg/L with TSAT <20%2
Most of the evidence refers to patients with left ventricular ejection fraction ≤45%3
KDIGO guidelines (2025) for patients with CKD4

The KDIGO 2025 clinical practice guideline for iron deficiency anaemia in CKD
makes the following recommendations for the use of iron to treat anaemia in CKD:
- In people with anaemia and CKD not receiving dialysis or treated with peritoneal dialysis (CKD G5PD), we suggest initiating iron if:
- ferritin <100 ng/ml (<100 μg/l) and transferrin saturation (TSAT) <40%, or
- ferritin ≥100 ng/ml (≥100 μg/l) and <300 ng/ml (<300 μg/l), and TSAT <25%
- In people with anaemia and CKD not receiving a haemodialysis in whom iron is initiated, we suggest using either oral or IV iron based on the person’s values and preferences
NICE guidelines (NG203; 2021) for patients with CKD5

- Consider investigating and managing anaemia in adults, children and young people with CKD if:
- their haemoglobin (Hb) level falls to 110 g/litre or less (or 105 g/litre or less if younger than 2 years) or
- they develop symptoms attributable to anaemia
- ESA (erythropoietic stimulating agent) therapy should not be started in the presence of absolute ID without also managing the ID
Iron therapy for people who are iron deficient and not on ESA therapy:
- Offer iron therapy to adults, children and young people with anaemia of CKD who are iron deficient and who are not receiving ESA therapy, before discussing ESA therapy
- Discuss the risks and benefits of treatment options. Take into account the person's choice
- For people who are not having haemodialysis, consider a trial of oral iron before offering IV iron therapy. If they are intolerant of oral iron or target Hb levels are not reached within 3 months, offer IV iron therapy
- For people who are having haemodialysis, offer IV iron therapy
Iron therapy for people who are iron deficient and receiving ESA therapy:
- Offer iron therapy to adults, children and young people with anaemia of CKD who are iron deficient and who are receiving ESA therapy
- Discuss the risks and benefits of treatment options. Take into account the person's choice
- For adults and young people, offer IV iron therapy
- For children who are having haemodialysis, offer IV iron therapy
For children who are not having haemodialysis, consider oral iron. If the child is intolerant of oral iron or target Hb levels are not reached within 3 months, offer IV iron therapy
Ferinject is approved for children and adolescents aged 1 to 13 years and adults and adolescents aged 14 years and older. The efficacy and safety of Ferinject have not been investigated in children below 1 year of age. Ferinject is therefore not recommended for use in children in this age group.1
In children aged 1 to 13 years with CKD requiring haemodialysis, the efficacy and safety of Ferinject have not been investigated. Ferinject is therefore not recommended for use in children aged 1 to 13 years with CKD requiring haemodialysis.1
NICE guidelines (NG24; 2015) for patients with pre-operative anaemia6

- Offer oral iron before and after surgery to patients with IDA
- Consider IV iron before or after surgery for patients who:
- have IDA and cannot tolerate or absorb oral iron, or are unable to adhere to oral iron treatment
- are diagnosed with functional ID
- are diagnosed with IDA, and the interval between the diagnosis of anaemia and surgery is predicted to be too short for oral iron to be effective
PRODIGGEST guidelines (2017, AEG) for patients with GI bleeding7

- See appropriate resuscitation and management guidelines in the case of acute bleeding and/or haemodynamic instability
- IV iron replacement therapy should be considered to avoid transfusion where appropriate (as part of a wider patient blood management strategy)
- Indications for IV iron in patients with GI bleeding while in hospital:
- Need for rapid correction of anaemia (moderate-severe anaemia) are diagnosed with functional ID
- Need for invasive surgery with a risk of significant bleeding
- Need for ESAs in order to prevent the primary cause of non-response to erythropoietin, which is functional ID
- Need for artificial feeding
- As a possible alternative to allogenic blood transfusion when this is not accepted
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
ADR, adverse drug reaction; AEG, Asociación Española de Gastroenterología (Spanish Association of Gastroenterology); CKD, chronic kidney disease; ESA, erythropoiesis-stimulating agent, ESC, European Society of Cardiology; GI, gastointestinal; Hb, haemoglobin; HF, heart failure; HFmrEF, heart failure with mildly-reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; ID, iron deficiency; IDA, iron deficiency anaemia; IV, intravenous; KDIGO, Kidney Disease Improving Global Outcomes; NICE, National Institute for Health and Care Excellence; PRODIGGEST, healthcare PROtocols to improve interDIsciplinary manaGEment of gaSTrointestinal diseases in hospital settings; QoL, quality of life; TSAT, transferrin saturation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- McDonagh TA, et al. Eur Heart J 2021;42(36):3599–3726 (+ suppl).
- McDonagh TA, et al. Eur Heart J 2023;00:1–13.
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO 2025 clinical practice guideline for anemia in chronic kidney disease (CKD). Public review draft, November 2024. Available at: kdigo.org. Accessed September 2025.
- National Institute for Health and Care Excellence (NICE). NICE guideline 203. Chronic kidney disease: assessment and management, August 2021 (last updated November 2021). Available at: www.nice.org.uk. Accessed September 2025.
- National Institute for Health and Care Excellence (NICE). NICE guideline 24. Blood transfusion, November 2015. Available at: www.nice.org.uk. Accessed September 2025.
- PRODIGGEST 2017: healthcare PROtocols to improve interDIsciplinary manaGEment of gaSTrointestinal diseases in hospital settings. Available at: www.aegastro.es. Accessed September 2025.
Resources
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
CKD, chronic kidney disease; HF, heart failure.
References
- Ferinject SmPC. Available at: www.medicines.org.uk.
Additional
DE-FCM-2100195
Efficacy and Safety
Ferinject for iron deficiency in patients receiving treatment for cancer
Key learning points:
- Intravenous iron can be used in line with the ESMO guidelines to treat iron deficiency anaemia in patients with cancer2
- Ferinject can correct iron deficiency anaemia in patients with cancer receiving chemotherapy, with or without an ESA3
Treatments such as blood transfusion and oral iron offer benefits to patients with cancer and may be appropriate for certain patients, but there are limitations to consider4–8

Blood transfusion4
- Blood supply issues
- Cost to NHS
- Inherent transfusion risk
- Adverse transfusion events

Oral iron
- The absorption of oral iron may be blocked by raised hepcidin levels due to systemic inflammation5
- Blood loss in patients with cancer may exceed their capacity to absorb oral iron6
- The average body absorbs 1–2 mg/day of iron via the gut, oral iron absorption capacity is limited and may not be adequate for the needs of the body7
- Side effects may result in non-adherence and treatment failure8
ESMO IV iron recommendations for patients with cancer and iron deficiency (ID) anaemia2
Absolute iron deficiency2
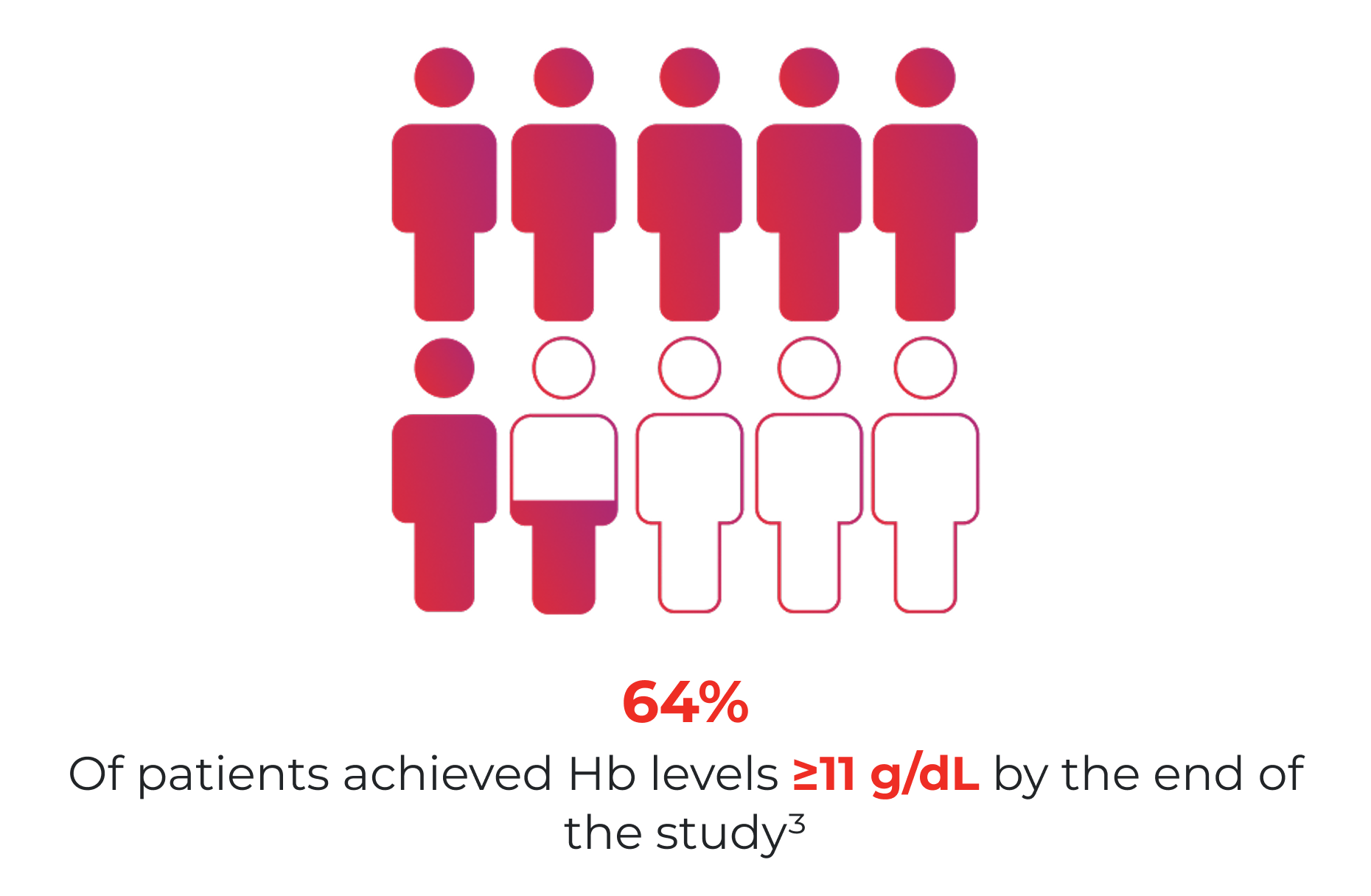
“Patients receiving ongoing ChT who present with anaemia (Hb ≤11 g/dL or Hb decrease ≥2 g/dL from a baseline level ≤12 g/dL) and absolute ID (serum ferritin <100 ng/mL) should receive iron treatment with an IV iron preparation to correct ID.”
Functional iron deficiency2
“IV iron without additional anaemia therapy may be considered in patients with functional ID (TSAT <20% and serum ferritin >100 ng/mL).”
Ferinject can correct iron deficiency anaemia in patients with cancer receiving chemotherapy, with or without an ESA3
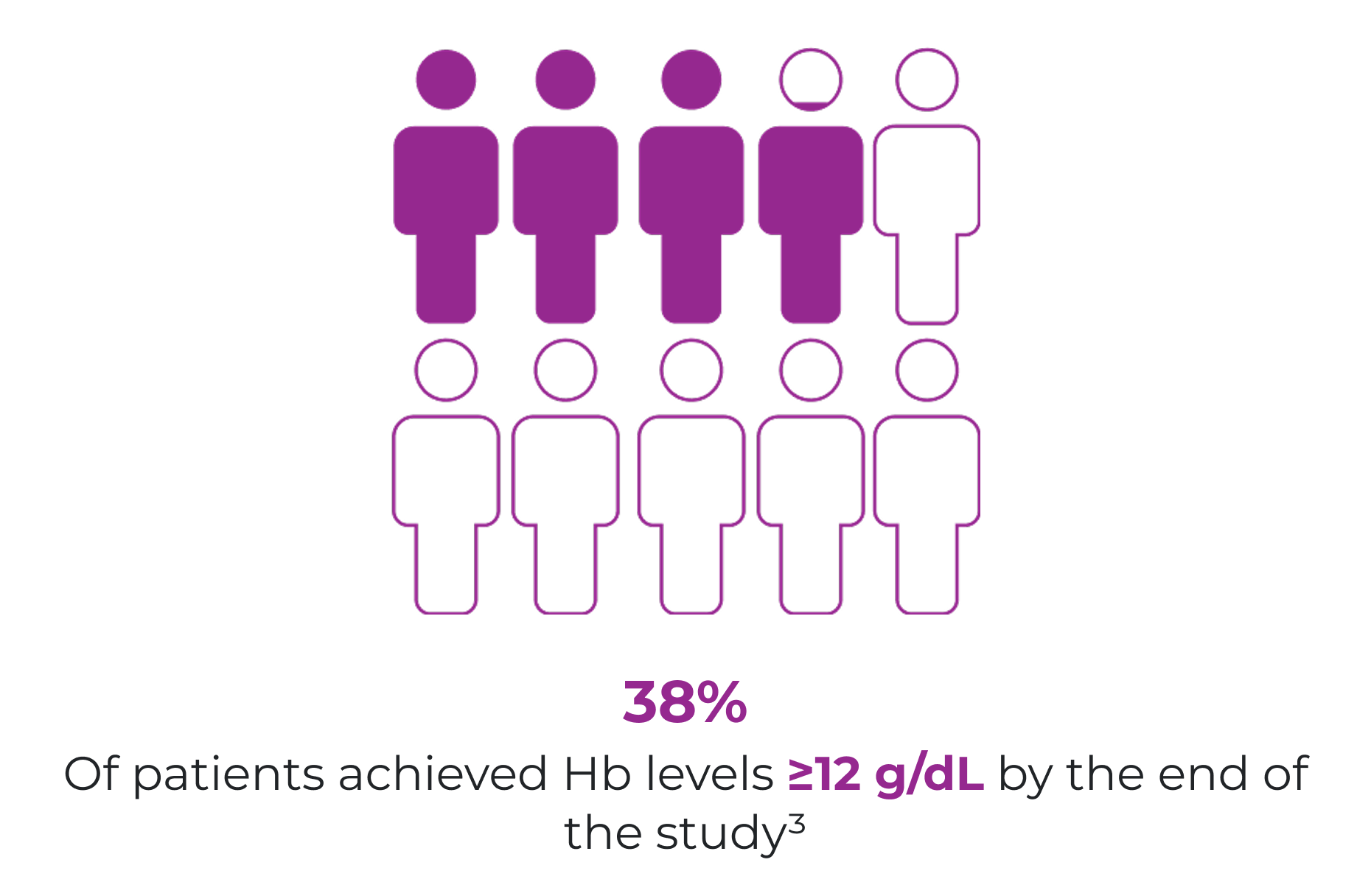
An observational study of 639 patients with cancer showed a substantial Hb increase and stabilisation at 11‑12 g/dL with Ferinject, with or without an ESA (n=420 effectiveness population).3
Study design and endpointsStudy baseline3
10 g/dL
Median Hb level in all censored patients
Week 5+3
11–12 g/dL
Median Hb level in all patients, with or without ESA
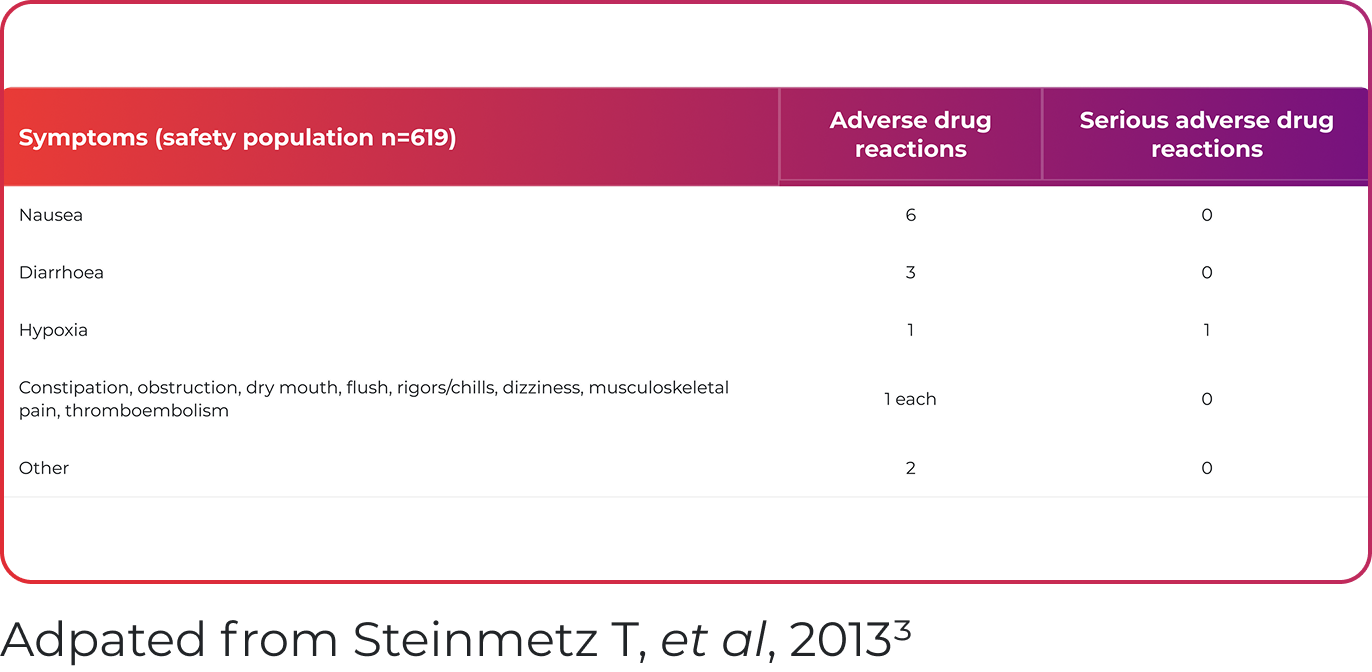
Ferinject was well tolerated, with 2.3% of patients reporting putative drug-related adverse events3

Long-term safety of IV iron in oncology is not yet fully established2

Learn more about how iron deficiency and iron deficiency anaemia affects your patients receiving treatment for cancer
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated

Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
ADR, adverse drug reaction; ChT, chemotherapy; ESA, erythropoiesis-stimulating agent; ESMO, European Society for Medical Oncology; Hb, haemoglobin; ID, iron deficiency; NHS, National Health Service; TSAT, transferrin saturation
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- Aapro M, et al. Ann Oncol 2018;29:iv96–iv110.
- Steinmetz T, et al. Ann Oncol 2013;24:475–482.
- Hofman A, et al. Oncologist 2011;16 Suppl 3:3–11.
- Hentze M, et al. Cell 2010;142:24–38.
- Aapro M, et al. Ann Oncol 2012;23:1954–1962.
- Moretti D, et al. Blood 2015;126:1981–1989.
- Tolkien Z, et al. PLoS One 2015;10:e0117383.
Iron Deficiency Anaemia in Oncology
Iron deficiency in patients receiving treatment for cancer
Key learning points:
- Anaemia is common in patients receiving cancer treatment2,3
- The symptomatic burden of anaemia in patients with cancer has a detrimental effect on quality of life4
- Iron deficiency anaemia is a modifiable comorbidity in patients with cancer and it can be treated2
Anaemia is a common comorbidity in patients with cancer2,3
In the European Cancer Anaemia Survey (ECAS):

14,912
patients with cancer were evaluated for up to 6 months3 (15,367 enrolled)

39.3%
of patients presented with anaemia3*
(n=5,706/14,520)
67%
of patients had anaemia* at some point during the study3 (n=9,131/13,628)

39.3%
of patients with anaemia had a Hb <10 g/dL3
(n=5,860/14,912)
Patients receiving chemotherapy had the highest incidence of anaemia vs patients who did not receive any treatment, patients receiving radiotherapy and patients receiving concomitant chemo-radiotherapy3
(62.7% (n=3,301/5,265) vs 41.9% (n=2,206/5,265)
and 19.5% (n=1,027/5,265), respectively)
The incidence of anaemia increased with subsequent chemotherapy cycles3
(19.5% (n=533/2,732) in first cycle vs 46.7%
(n=1,276/2,732) in fifth cycle)
*Anaemia was defined as Hb <12.0 g/dL.3
Burden of anaemia in cancer
Anaemia in cancer may have consequences for patient prognosis:
Tumour response5
In patients with anaemia, low Hb levels may impact chemotherapy response rate in breast cancer
Progression‑free survival (PFS)6
Anaemia was associated with poor PFS in patients with prostate cancer
Overall survival (OS)7
Post‑diagnosis Hb change may be a predictor of OS in patients with lung, breast, colorectal and liver cancer in patients with anaemia
Mortality8
There may be an overall estimated 65% increase in the risk of mortality in patients with cancer and anaemia vs those without anaemia
Anaemia in cancer is complex and multifactorial but functional iron deficiency is one of the main contributors9
Common causes of iron deficiency anaemia in patients with cancer:
ESMO guidelines for assessing iron status in patients with cancer1Absolute iron deficiency9
Total body iron depleted with low iron stores
Caused by:
- poor iron intake
- impaired absorption
- acute and chronic blood loss (e.g. surgery)
Functional iron deficiency9
Inability to access iron stores
Caused by:
- increased erythropoiesis (through blood loss or use of ESAs)
- systemic inflammation (which increases hepcidin levels)

Reduction of iron supply to all cells of the body

Iron deficiency or iron deficiency anaemia
Other causes of anaemia in cancer include:9
- vitamin B12 and folate deficiency
- chemotherapy-induced anaemia (bone marrow and renal toxicity, red cell haemolysis)
- tumour-induced anaemia (bone marrow metastasis)
Ferinject is only indicated for the treatment of anaemia caused by iron deficiency1
Multiple factors are associated with iron deficiency and anaemia in oncology10
A prospective study of 1,528 patients with cancer investigated the interdependencies between iron deficiency and various clinical parameters.10
Both iron deficiency and anaemia are more common at a more advanced stage of disease at diagnosis (p<0.001)
Iron deficiency and anaemia were most common in patients who had recently received their last anticancer therapy within 12 weeks vs those who had received it more than 12 weeks from baseline (p<0.001)
Patients with persistent or progressive disease were most likely to have iron deficiency or anaemia vs patients in complete remission (p<0.001)
Both iron deficiency and anaemia significantly correlated with poor ECOG performance status (p=0.005 and p=0.001, respectively)
The symptomatic burden of anaemia in patients with cancer has a detrimental effect on quality of life4
Anaemia is associated with symptoms of fatigue, lethargy, dyspnoea, loss of appetite and inability to concentrate, which may have a negative impact on quality of life.4
In a study of patients with cancer and anaemia (n=179), there were significant positive relationships between Hb levels and...4
6 of 6
…domains of the Functional Assessment of Cancer Therapy – Anaemia†

6 of 8
…of the SF-36 domains†
†FACT‑G, fatigue, non‑fatigue, anaemia, FACT‑F and FACT‑An.4
‡Physical function, role physical, social functioning, mental health, energy/vitality, health perception. Differences in pain and role mental were not significant.4
Iron deficiency management in patients with cancer
- Early anaemia management may help patients maintain Hb target levels during chemotherapy3
- Iron deficiency in anaemia is a modifiable comorbidity in patients with cancer and it can be treated2
- Raising Hb level may improve the quality of life of patients with cancer and anaemia4
Learn more about the treatment of iron deficiency and iron deficiency anaemia in patients with cancer
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
ADR, adverse drug reaction; ECAS, European Cancer Anaemia Survey; ECOG, Eastern Cooperative Oncology Group; ESA, erythropoiesis-stimulating agents; ESMO, European Society for Medical Oncology; FACT, Functional Assessment of Cancer Therapy; Hb, haemoglobin; OS, overall survival; PFS, progression free survival; SF-36, short form-36; TSAT, transferrin saturation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- Aapro, et al. Ann Oncol 2018;29:iv96–iv110.
- Ludwig H, et al. Eur J Cancer 2004;40:2293–2306.
- Lind M, et al. Br J Cancer 2002;86:1243–1249.
- Bottini A, et al. Br J Cancer 2003;89:977–982.
- Dai D, et al. Am J Transl Res 2018;10:3877–3886.
- Wan S, et al. BMC Cancer 2013;13:340.
- Caro J J, et al. Cancer 2001;91:2214–2221.
- Busti F, et al. Pharmaceuticals (Basel) 2018;11:94.
- Ludwig H, et al. Ann Oncol 2013;24:1886–1892.
Efficacy and Safety
AFFIRM-AHF highlights the potential of Ferinject® (ferric carboxymaltose) treatment of iron deficiency to improve outcomes in patients with heart failure
AFFIRM-AHF is the first international, multicentre, double-blind, randomised placebo-controlled trial to compare the effect of Ferinject with placebo on heart failure (HF) re-hospitalisations and cardiovascular (CV) mortality in patients with iron deficiency who were stabilised after an episode of acute HF (N=1,108).2
The study supports the use of Ferinject in patients stabilised for acute HF with concomitant iron deficiency and a left ventricular ejection fraction ≤50%2
AFFIRM-AHF outcomes were evaluated up to 52 weeks after randomisation:2
Primary Endpoint
- Composite of total re-hospitalisations for HF and CV death up to 52 weeks after randomisation
Secondary Endpoint
- Total HF re-hospitalisations
- CV death
- Composite of total CV re-hospitalisations and CV death
- Time to first HF re-hospitalisation or CV death
- Days lost due to HF re-hospitalisations or CV death
AFFIRM-AHF study design and visit schedule
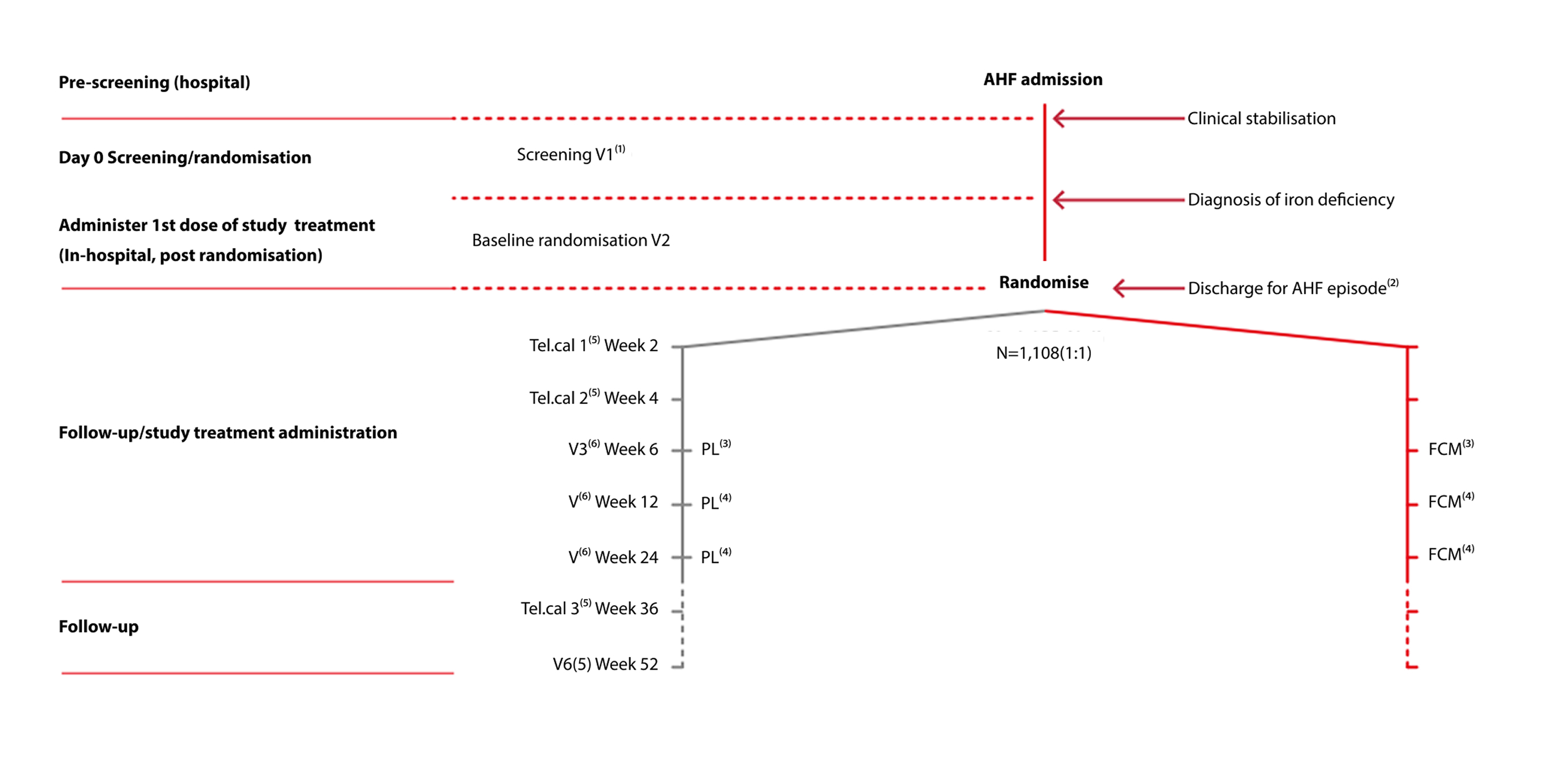
Adapted from Ponikowski P et al. 2020.2
- Performed in hospital during the AHF admission (‘Index hospitalisation’)
- Index hospitalisation discharge after administration of study treatment at the discretion of the investigator
- Second dose of study treatment (repletion phase) done at the outpatient visit
- Study treatment to be administered only if iron deficiency persists
- Telephone contact
- Outpatient clinic visit.

Register now with CSL Vifor to be informed of new webinars delivered by experts in the field of cardiology and nephrology. This will include promotional content.
Ferinject numerically reduced the risk for the composite endpoint of HF re-hospitalisations and CV death, in patients with iron deficiency and LVEF ≤50% stabilised after an episode of acute HF, although statistical significance was narrowly missed (P=0.059)
PRIMARY ENDPOINT: TOTAL HF RE-HOSPITALISATIONS AND CV DEATH
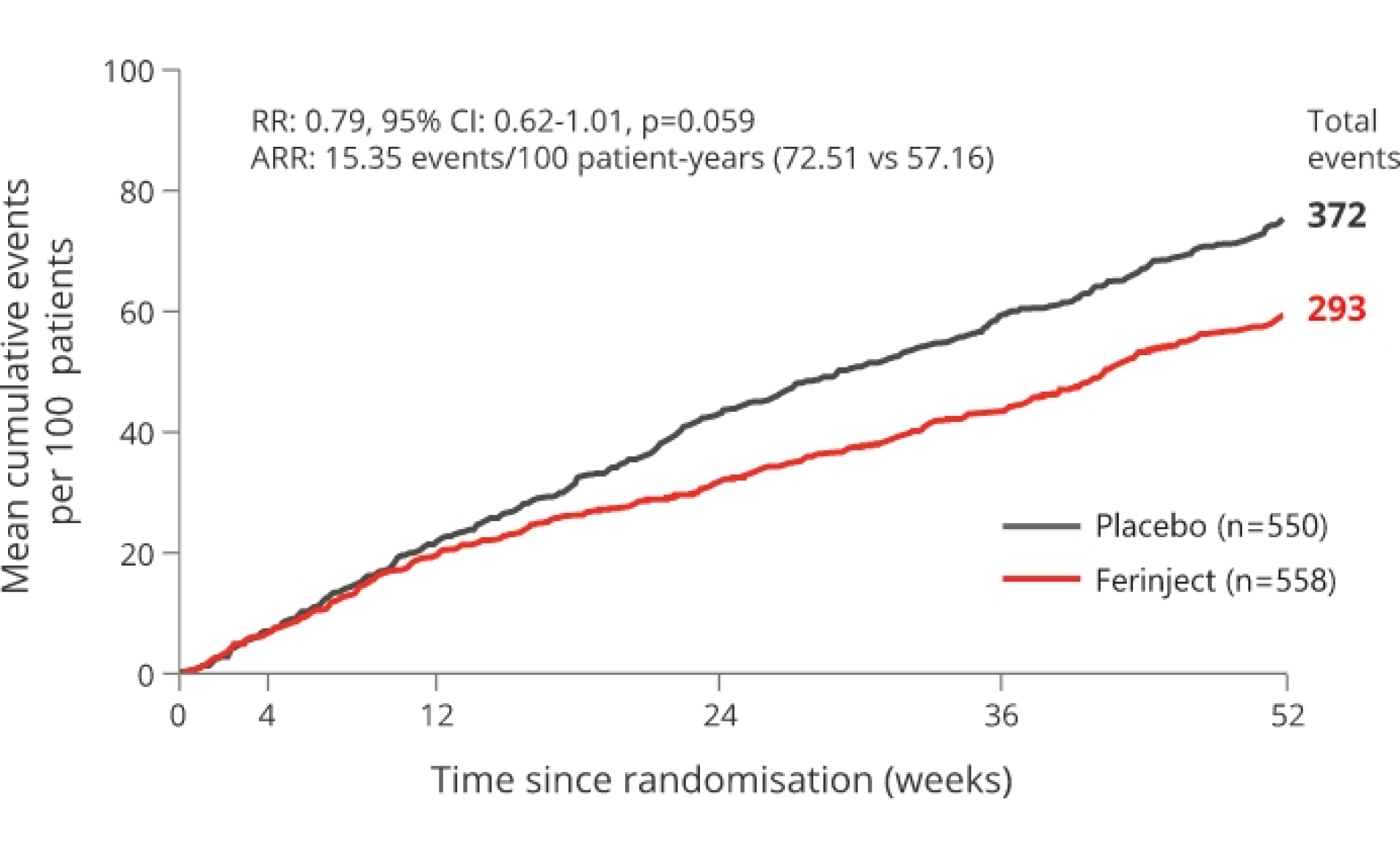
21% relative risk reduction p=0.059 (not significant). Primary endpoint not met
*mITT population.
Adapted from Ponikowski P et al, 2020.2
The primary endpoint was driven by a 26% reduction in HF re-hospitalisation with Ferinject versus placebo with no apparent effect on CV death.2
SECONDARY ENDPOINT: TOTAL HF RE-HOSPITALISATIONS
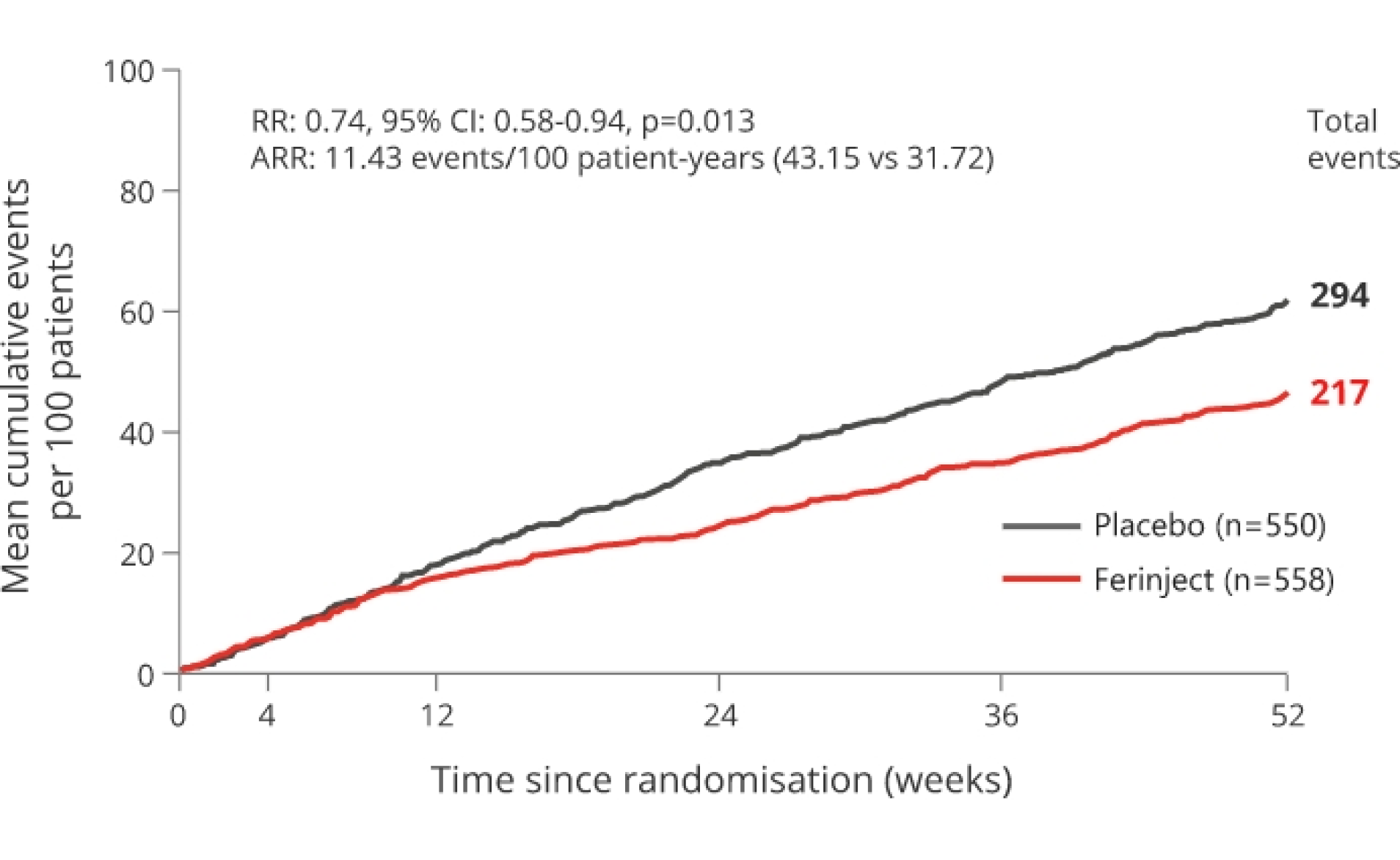
26% relative risk reduction vs placebo p=0.013
*mITT population.
Adapted from Ponikowski P et al, 2020.2
Treatment benefits with Ferinject® (ferric carboxymaltose), versus placebo across secondary outcomes2
First heart failure hospitalisation or CV death occurred in 181/558 (32%) of patients in the Ferinject group versus in 209/550 (38%) of the patients in the placebo group
- ARR: 9.7 events/100 patient-years, HR: 0.80, 95% CI: 0.66-0.98, p=0.030
Days lost due to HF hospitalisations and CV death with Ferinject were reduced by 33% versus placebo
- Rate per 100 patient-years: 369 days in the Ferinject group versus 548.4 days in the placebo group
- ARR: 179.4 days/100 patient-years, RR: 0.67, 95% CI 0.47-0.97, p=0.035
No statistically significant treatment effects in other secondary outcomes
- 20% reduction in total CV hospitalisations and CV death with Ferinject versus placebo
- ARR: 19.09 events/100 patient-years, RR: 0.80, 95% CI: 0.64-1.00, p=0.050
- Similar CV deaths in the Ferinject and placebo groups
- ARR: 0.2 events/100 patient-years, HR: 0.96, 95% CI: 0.70-1.32, p=0.81
Pre-specified pre-COVID-19 sensitivity analysis
The AFFIRM-AHF study included a pre-specified pre-COVID-19 sensitivity analysis; patient follow-up in each participating country was censored at the date when its first COVID-19 patient was reported.2
The sensitivity analysis suggested a statistically significant, 25% relative risk reduction with Ferinject versus placebo on the combined endpoint of HF hospitalisation and CV death2
- Rate per 100 patient-years: 55.24 versus 73.48, ARR: 18.24 events/100 patient-years, RR: 0.75, 95% CI: 0.59-0.96, p=0.024
AFFIRM-AHF added to the well-characterised tolerability profile of Ferinject1–3
Overall incidence of adverse events (AEs), serious AEs, and AEs leading to hospitalisation, withdrawal of treatment, or study discontinuation were similar in the Ferinject and placebo groups.2
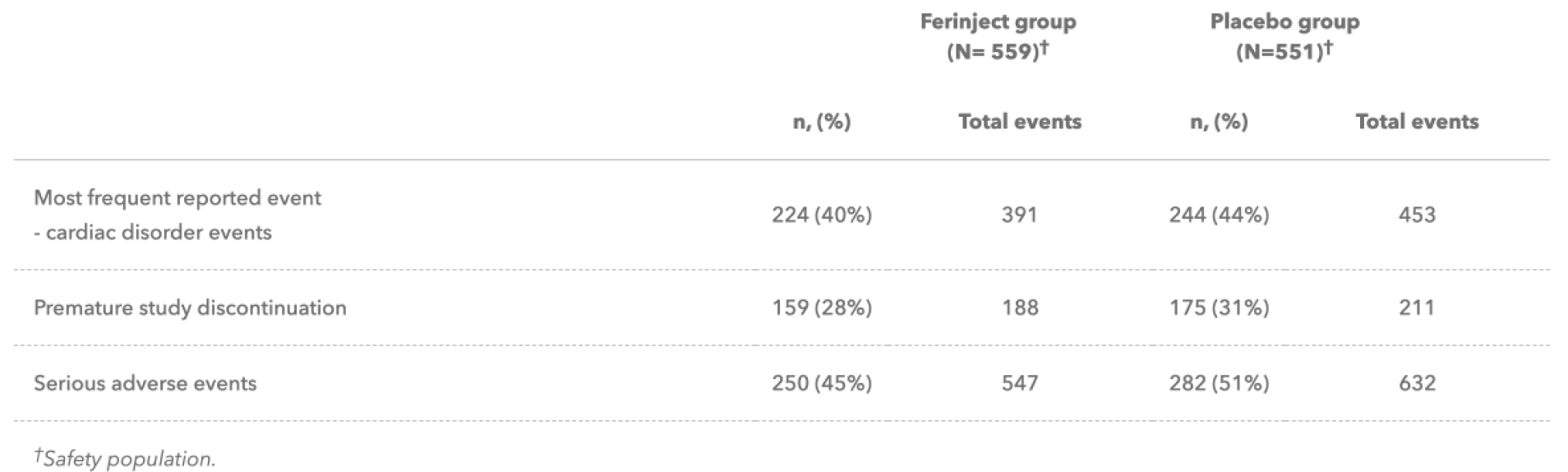
Ferinject has a well-characterised tolerability profile:1
- The most commonly reported adverse drug reaction (ADR) is nausea (3.2%), followed by injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Injection/infusion site reactions comprise several ADRs which individually are either uncommon or rare
- The most serious ADR is anaphylactic reactions (rare ≥1/10,000 to <1/1000); fatalities have been reported. Ferinject has a documented frequency of serious hypersensitivity reactions of ≥1/1,00 to <1/100. There have been reports of hypersensitivity reactions which progressed to Kounis syndrome (acute allergic coronary arteriospasm that can result in myocardial infarction)
- Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each Ferinject administration
- If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardio respiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution
- Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information
Find out moreReferences & footnotes
Footnotes
ADR, adverse drug reaction; AE, adverse event; AHF, acute heart failure; ARR, absolute risk reduction; CI, confidence interval; CV, cardiovascular; FCM, ferric carboxymaltose; HF, heart failure; HR, hazard ratio; PL, placebo; RR, rate ratio; V, visit.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- Ponikoski P, et al Lancet 2020 Dec 12;396(10266):1895-1904. doi: 10.1016/S0140-6736(20)32339-4.
- McDonagh T, et al. Eur J Heart Fail 2018;20(12):1664–1672.
Efficacy and Safety
AFFIRM-AHF highlights the potential of Ferinject® (ferric carboxymaltose) treatment of iron deficiency to improve outcomes in patients with heart failure
AFFIRM-AHF is the first international, multicentre, double-blind, randomised placebo-controlled trial to compare the effect of Ferinject with placebo on heart failure (HF) re-hospitalisations and cardiovascular (CV) mortality in patients with iron deficiency who were stabilised after an episode of acute HF (N=1,108).2
The study supports the use of Ferinject in patients stabilised for acute HF with concomitant iron deficiency and a left ventricular ejection fraction ≤50%2
AFFIRM-AHF outcomes were evaluated up to 52 weeks after randomisation:2
Primary Endpoint
- Composite of total re-hospitalisations for HF and CV death up to 52 weeks after randomisation
Secondary Endpoint
- Total HF re-hospitalisations
- CV death
- Composite of total CV re-hospitalisations and CV death
- Time to first HF re-hospitalisation or CV death
- Days lost due to HF re-hospitalisations or CV death
AFFIRM-AHF study design and visit schedule

Adapted from Ponikowski P et al. 2020.2
- Performed in hospital during the AHF admission (‘Index hospitalisation’)
- Index hospitalisation discharge after administration of study treatment at the discretion of the investigator
- Second dose of study treatment (repletion phase) done at the outpatient visit
- Study treatment to be administered only if iron deficiency persists
- Telephone contact
- Outpatient clinic visit.

Register now with CSL Vifor to be informed of new webinars delivered by experts in the field of cardiology and nephrology. This will include promotional content.
Ferinject numerically reduced the risk for the composite endpoint of HF re-hospitalisations and CV death, in patients with iron deficiency and LVEF ≤50% stabilised after an episode of acute HF, although statistical significance was narrowly missed (P=0.059)
PRIMARY ENDPOINT: TOTAL HF RE-HOSPITALISATIONS AND CV DEATH

21% relative risk reduction p=0.059 (not significant). Primary endpoint not met
*mITT population.
Adapted from Ponikowski P et al, 2020.2
The primary endpoint was driven by a 26% reduction in HF re-hospitalisation with Ferinject versus placebo with no apparent effect on CV death.2
SECONDARY ENDPOINT: TOTAL HF RE-HOSPITALISATIONS

26% relative risk reduction vs placebo p=0.013
*mITT population.
Adapted from Ponikowski P et al, 2020.2
Treatment benefits with Ferinject® (ferric carboxymaltose), versus placebo across secondary outcomes2
First heart failure hospitalisation or CV death occurred in 181/558 (32%) of patients in the Ferinject group versus in 209/550 (38%) of the patients in the placebo group
- ARR: 9.7 events/100 patient-years, HR: 0.80, 95% CI: 0.66-0.98, p=0.030
Days lost due to HF hospitalisations and CV death with Ferinject were reduced by 33% versus placebo
- Rate per 100 patient-years: 369 days in the Ferinject group versus 548.4 days in the placebo group
- ARR: 179.4 days/100 patient-years, RR: 0.67, 95% CI 0.47-0.97, p=0.035
No statistically significant treatment effects in other secondary outcomes
- 20% reduction in total CV hospitalisations and CV death with Ferinject versus placebo
- ARR: 19.09 events/100 patient-years, RR: 0.80, 95% CI: 0.64-1.00, p=0.050
- Similar CV deaths in the Ferinject and placebo groups
- ARR: 0.2 events/100 patient-years, HR: 0.96, 95% CI: 0.70-1.32, p=0.81
Pre-specified pre-COVID-19 sensitivity analysis
The AFFIRM-AHF study included a pre-specified pre-COVID-19 sensitivity analysis; patient follow-up in each participating country was censored at the date when its first COVID-19 patient was reported.2
The sensitivity analysis suggested a statistically significant, 25% relative risk reduction with Ferinject versus placebo on the combined endpoint of HF hospitalisation and CV death2
- Rate per 100 patient-years: 55.24 versus 73.48, ARR: 18.24 events/100 patient-years, RR: 0.75, 95% CI: 0.59-0.96, p=0.024
AFFIRM-AHF added to the well-characterised tolerability profile of Ferinject1–3
Overall incidence of adverse events (AEs), serious AEs, and AEs leading to hospitalisation, withdrawal of treatment, or study discontinuation were similar in the Ferinject and placebo groups.2

Ferinject has a well-characterised tolerability profile:1
- The most commonly reported adverse drug reaction (ADR) is nausea (3.2%), followed by injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Injection/infusion site reactions comprise several ADRs which individually are either uncommon or rare
- The most serious ADR is anaphylactic reactions (rare ≥1/10,000 to <1/1000); fatalities have been reported. Ferinject has a documented frequency of serious hypersensitivity reactions of ≥1/1,00 to <1/100. There have been reports of hypersensitivity reactions which progressed to Kounis syndrome (acute allergic coronary arteriospasm that can result in myocardial infarction)
- Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each Ferinject administration
- If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardio respiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution
- Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information
Find out moreReferences & footnotes
Footnotes
ADR, adverse drug reaction; AE, adverse event; AHF, acute heart failure; ARR, absolute risk reduction; CI, confidence interval; CV, cardiovascular; FCM, ferric carboxymaltose; HF, heart failure; HR, hazard ratio; PL, placebo; RR, rate ratio; V, visit.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- Ponikoski P, et al Lancet 2020 Dec 12;396(10266):1895-1904. doi: 10.1016/S0140-6736(20)32339-4.
- McDonagh T, et al. Eur J Heart Fail 2018;20(12):1664–1672.
Efficacy and Safety
Anaemia, with or without iron deficiency, is common in pre-operative patients2
The National Comparative Audit of Blood Transfusion evaluated the percentage of anaemic patients by surgical group and found that anaemia is present in 33%-77% of patients across a broad a range of surgical therapy areas.2
Associative retrospective studies have suggested worse outcomes in surgical patients with anaemia compared with those without anaemia3,4
Hospital Stay
22% longer hospital stay (associative retrospective studies)
Mean duration: 11 days for patients with anaemia (n=2,090) vs 9 days for patients without anaemia (n=2,090), p=0.00013

Infections
1.93x higher chance of infection (associative retrospective studies)
OR: 1.93, patients with anaemia (n=12,425) vs patients without anaemia (n=11,376), p=0.014

Mortality
2.90x higher chance of 30-day mortality (associative retrospective studies)
OR: 2.90, patients with anaemia (n=14,978) vs patients without anaemia (n=7,430), p<0.0014

Acute kidney injury
22% longer hospital stay (associative retrospective studies)
OR: 3.75, patients with anaemia (n=1,576) vs patients without anaemia (n=1,035), p<0.0014
RBC transfusion
5.04x higher chance of RBC transfusion (associative retrospective studies)
OR: 5.04, patients with anaemia (n=15,804) vs patients without anaemia (n=9,539), p<0.0014
- Hospital stay: determined from a retrospective single-centre cohort study of patients aged >18 years undergoing non-cardiac surgery between March 2003 and June 2006 (N=7,760).3
- Infection, mortality, kidney injury and transfusion rates: determined from a systematic review and meta-analysis of observational studies exploring associations between pre-operative anaemia and postoperative outcomes (24 studies, N=949,445).4
Iron deficiency (ID) is one of the most common causes of anaemia5
Approximately 50% of pre-operative anaemia cases are attributable to ID, which can have absolute or functional causes.5
ID and iron deficiency anaemia (IDA) management is a key element in patient blood management6,7
It is important to assess and treat IDA as soon as possible prior to surgery. 8,9
Diagnosis
Expert consensus recommends the assessment of iron status at least 30 days before surgery10
Supported by NHS guidance11
Treatment choice
Iron supplementation is recommended in scheduled surgery for the correction of IDA8,9,12
View the NICE guidance on the pre-operative use of iron (NG24: blood transfusion)
NICE guideline 24 (NG24)12
Offer oral iron before and after surgery to patients with IDA. Consider intravenous iron before or after surgery for patients who:
- have IDA and cannot tolerate or absorb oral iron, or are unable to adhere to oral iron treatment
- are diagnosed with functional ID
- are diagnosed with IDA, and the interval between the diagnosis of anaemia and surgery is predicted to be too short for oral iron to be effective
Ferinject has a well-characterised tolerability profile:1
- The most commonly reported adverse drug reaction (ADR) is nausea (3.2%), followed by injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Injection/infusion site reactions comprise several ADRs which individually are either uncommon or rare
- The most serious ADR is anaphylactic reactions (rare ≥1/10,000 to <1/1000); fatalities have been reported. Ferinject has a documented frequency of serious hypersensitivity reactions of ≥1/1,000 to <1/100. There have been reports of hypersensitivity reactions which progressed to Kounis syndrome (acute allergic coronary arteriospasm that can result in myocardial infarction)
- Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each Ferinject administration
- If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardio respiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution
- Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information
Find out moreReferences & footnotes
Footnotes
ADR, adverse drug reaction; ID, iron deficiency; IDA, iron deficiency anaemia; OR, odds ratio; RBC, red blood cell
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- National Comparative Audit of Blood Transfusion. 2015 audit of patient blood management in adults undergoing elective, scheduled surgery. Available at: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/14905/2015-pbm-in- scheduled-surgery-audit-report.pdf. Accessed March 2025.
- Beattie W S, Karkouti K et al. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology 2009;110(3):574-581.
- Fowler A J, Ahmad T et al. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg 2015;102(11):1314-1324.
- Tibi P, McClure R S et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. Ann Thorac Surg 2021;112(3):981-1004.
- Clevenger B, Gurusamy K et al. Systematic review and meta-analysis of iron therapy in anaemic adults without chronic kidney disease: updated and abridged Cochrane review. Eur J Heart Fail 2016;18(7):774-785.
- National Blood Authority Australia. Three pillars of patient blood management. Available at: www.blood.gov.au. Accessed March 2025.
- Munoz M, Acheson A G et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017;72(2):233-247.
- Kozek-Langenecker S A, Ahmed A B et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol 2017;34(6):332-395.
- Beris P, Munoz M et al. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth 2008;100(5):599-604.
- Anaesthesia and perioperative medicine. GIRFT Programme national specialty report. Available at: www.gettingitrightfirsttime.co.uk. Accessed March 2025.
- National Institute for Health and Care Excellence (NICE). NICE guideline 24. Blood transfusion, November 2015. Available at: www.nice.org.uk/ guidance/ng24. Accessed March 2025.
Efficacy and Safety
Anaemia, with or without iron deficiency, is common in pre-operative patients2
The National Comparative Audit of Blood Transfusion evaluated the percentage of anaemic patients by surgical group and found that anaemia is present in 33%-77% of patients across a broad a range of surgical therapy areas.2
Associative retrospective studies have suggested worse outcomes in surgical patients with anaemia compared with those without anaemia3,4

Hospital Stay
22% longer hospital stay (associative retrospective studies)
Mean duration: 11 days for patients with anaemia (n=2,090) vs 9 days for patients without anaemia (n=2,090), p=0.00013

Infections
1.93x higher chance of infection (associative retrospective studies)
OR: 1.93, patients with anaemia (n=12,425) vs patients without anaemia (n=11,376), p=0.014

Mortality
2.90x higher chance of 30-day mortality (associative retrospective studies)
OR: 2.90, patients with anaemia (n=14,978) vs patients without anaemia (n=7,430), p<0.0014

Acute kidney injury
22% longer hospital stay (associative retrospective studies)
OR: 3.75, patients with anaemia (n=1,576) vs patients without anaemia (n=1,035), p<0.0014

RBC transfusion
5.04x higher chance of RBC transfusion (associative retrospective studies)
OR: 5.04, patients with anaemia (n=15,804) vs patients without anaemia (n=9,539), p<0.0014
- Hospital stay: determined from a retrospective single-centre cohort study of patients aged >18 years undergoing non-cardiac surgery between March 2003 and June 2006 (N=7,760).3
- Infection, mortality, kidney injury and transfusion rates: determined from a systematic review and meta-analysis of observational studies exploring associations between pre-operative anaemia and postoperative outcomes (24 studies, N=949,445).4
Iron deficiency (ID) is one of the most common causes of anaemia5
Approximately 50% of pre-operative anaemia cases are attributable to ID, which can have absolute or functional causes.5
ID and iron deficiency anaemia (IDA) management is a key element in patient blood management6,7
It is important to assess and treat IDA as soon as possible prior to surgery. 8,9
Diagnosis
Expert consensus recommends the assessment of iron status at least 30 days before surgery10
Supported by NHS guidance11
Treatment choice
Iron supplementation is recommended in scheduled surgery for the correction of IDA8,9,12
View the NICE guidance on the pre-operative use of iron (NG24: blood transfusion)
NICE guideline 24 (NG24)12
Offer oral iron before and after surgery to patients with IDA. Consider intravenous iron before or after surgery for patients who:
- have IDA and cannot tolerate or absorb oral iron, or are unable to adhere to oral iron treatment
- are diagnosed with functional ID
- are diagnosed with IDA, and the interval between the diagnosis of anaemia and surgery is predicted to be too short for oral iron to be effective
Ferinject has a well-characterised tolerability profile:1
- The most commonly reported adverse drug reaction (ADR) is nausea (3.2%), followed by injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Injection/infusion site reactions comprise several ADRs which individually are either uncommon or rare
- The most serious ADR is anaphylactic reactions (rare ≥1/10,000 to <1/1000); fatalities have been reported. Ferinject has a documented frequency of serious hypersensitivity reactions of ≥1/1,000 to <1/100. There have been reports of hypersensitivity reactions which progressed to Kounis syndrome (acute allergic coronary arteriospasm that can result in myocardial infarction)
- Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each Ferinject administration
- If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardio respiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution
- Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information
Find out moreReferences & footnotes
Footnotes
ADR, adverse drug reaction; ID, iron deficiency; IDA, iron deficiency anaemia; OR, odds ratio; RBC, red blood cell
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- National Comparative Audit of Blood Transfusion. 2015 audit of patient blood management in adults undergoing elective, scheduled surgery. Available at: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/14905/2015-pbm-in- scheduled-surgery-audit-report.pdf. Accessed March 2025.
- Beattie W S, Karkouti K et al. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology 2009;110(3):574-581.
- Fowler A J, Ahmad T et al. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg 2015;102(11):1314-1324.
- Tibi P, McClure R S et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. Ann Thorac Surg 2021;112(3):981-1004.
- Clevenger B, Gurusamy K et al. Systematic review and meta-analysis of iron therapy in anaemic adults without chronic kidney disease: updated and abridged Cochrane review. Eur J Heart Fail 2016;18(7):774-785.
- National Blood Authority Australia. Three pillars of patient blood management. Available at: www.blood.gov.au. Accessed March 2025.
- Munoz M, Acheson A G et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017;72(2):233-247.
- Kozek-Langenecker S A, Ahmed A B et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol 2017;34(6):332-395.
- Beris P, Munoz M et al. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth 2008;100(5):599-604.
- Anaesthesia and perioperative medicine. GIRFT Programme national specialty report. Available at: www.gettingitrightfirsttime.co.uk. Accessed March 2025.
- National Institute for Health and Care Excellence (NICE). NICE guideline 24. Blood transfusion, November 2015. Available at: www.nice.org.uk/ guidance/ng24. Accessed March 2025.
Efficacy and Safety
FIND-CKD: Ferinject treatment to achieve high ferritin levels in patients with chronic kidney disease and iron deficiency1,2
Key learning points
- Achieving higher ferritin levels with Ferinject in patients with iron deficiency and non-dialysis chronic kidney disease (ND-CKD) may lead to more effective IDA management vs achieving low ferritin levels with oral ferrous sulphate or intravenous (IV) Ferinject2
- Treating IDA with Ferinject to achieve high ferritin levels was not associated with an increased adverse event (AE) rate vs achieving low ferritin levels with Ferinject2
Results from the FIND-CKD study: treating iron deficiency to achieve high ferritin levels with Ferinject in patients with ND-CKD and IDA
FIND-CKD was a 56-week open-label, multi-centre, randomised-controlled, 3-arm study in 626 patients with CKD Stage 3–5 not yet on dialysis and with IDA. In this study, there were two Ferinject arms:
- Ferinject dosed to achieve ferritin levels of 400-600 mcg/L
- Ferinject dosed to achieve ferritin levels of 100-200 mcg/L; the comparator arm was oral ferrous sulphate dosed to maintain ferritin between 100 and 200 mcg/L
The primary endpoint was time to initiation of other anaemia management (erythropoiesis-stimulating agent (ESA), other iron therapy or blood transfusion) or occurrence of a haemoglobin (Hb) trigger of two consecutive values <10 g/dL during Weeks 8-52.2
FIND-CKD study design2
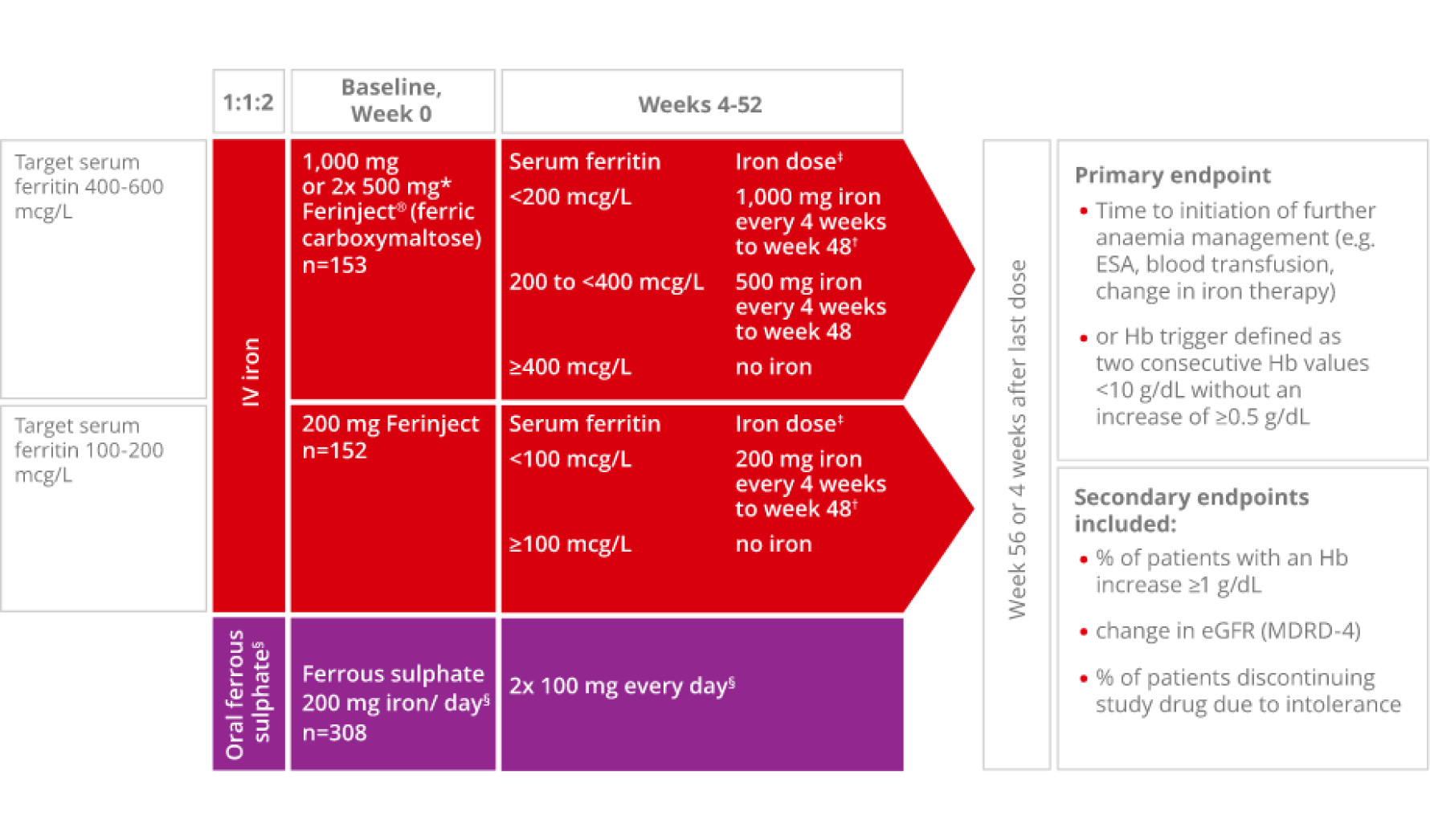
Adapted from Macdougall IC et al, 2014.2
*Patients ≤66 kg: 500 mg iron on Days 0 and 7.2
†Patients ≤66 kg: 500 mg iron on day of visit and 500 mg iron 1 week later.2
‡No administration if transferrin saturation level ≥40%.2
§Oral ferrous sulphate was withheld if serum ferritin was >200 mcg/L and restarted if/when serum ferritin was <100 mcg/L.2 The last dose of Ferinject was administered at Week 48, and the last dose of ferrous sulphate was administered at Week 52.2
In patients with IDA and ND-CKD, achieving high ferritin levels (400-600 mcg/L) with Ferinject significantly reduced and/or delayed the need for alternative anaemia management or the occurrence of an Hb trigger compared with achieving ferritin levels of 100-200 mcg/L with oral ferrous sulphate2
36/153 patients (23.5%) Ferinject vs 98/308 patients (31.8%) oral ferrous sulphate; HR: 0.65 (95% Cl: 0.44-0.95); p=0.026
The difference in the primary endpoint of time to initiation of an alternative treatment for anaemia or occurrence of a Hb trigger between Ferinject dosed to achieve high ferritin (400-600 mcg/L) and Ferinject dosed to achieve lower ferritin (100-200 mcg/L) was not statistically significant2
36/153 patients (23.5%) Ferinject high ferritin vs 49/153 patients (32.2%) Ferinject low ferritin; HR: 0.68 (95% Cl: 0.45-1.05); p=0.082
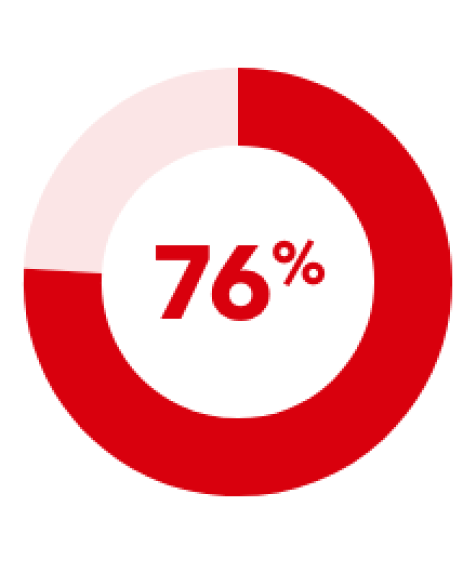
of patients with IDA and ND-CKD maintained a Hb level ≥10 g/dL or required no further anaemia treatment when treated with Ferinject dosed to achieve high ferritin levels of 400–600 mcg/L (n=117/153)2
The primary endpoint occurred in 36 patients (23.5%), 49 patients (32.2%) and 98 patients (31.8%) in the high-ferritin FCM, low-ferritin FCM and oral ferrous sulphate groups, respectively.2
In FIND-CKD, treating IDA with Ferinject dosed to achieve high ferritin levels was not associated with an increased AE rate vs achieving low ferritin levels with Ferinject2
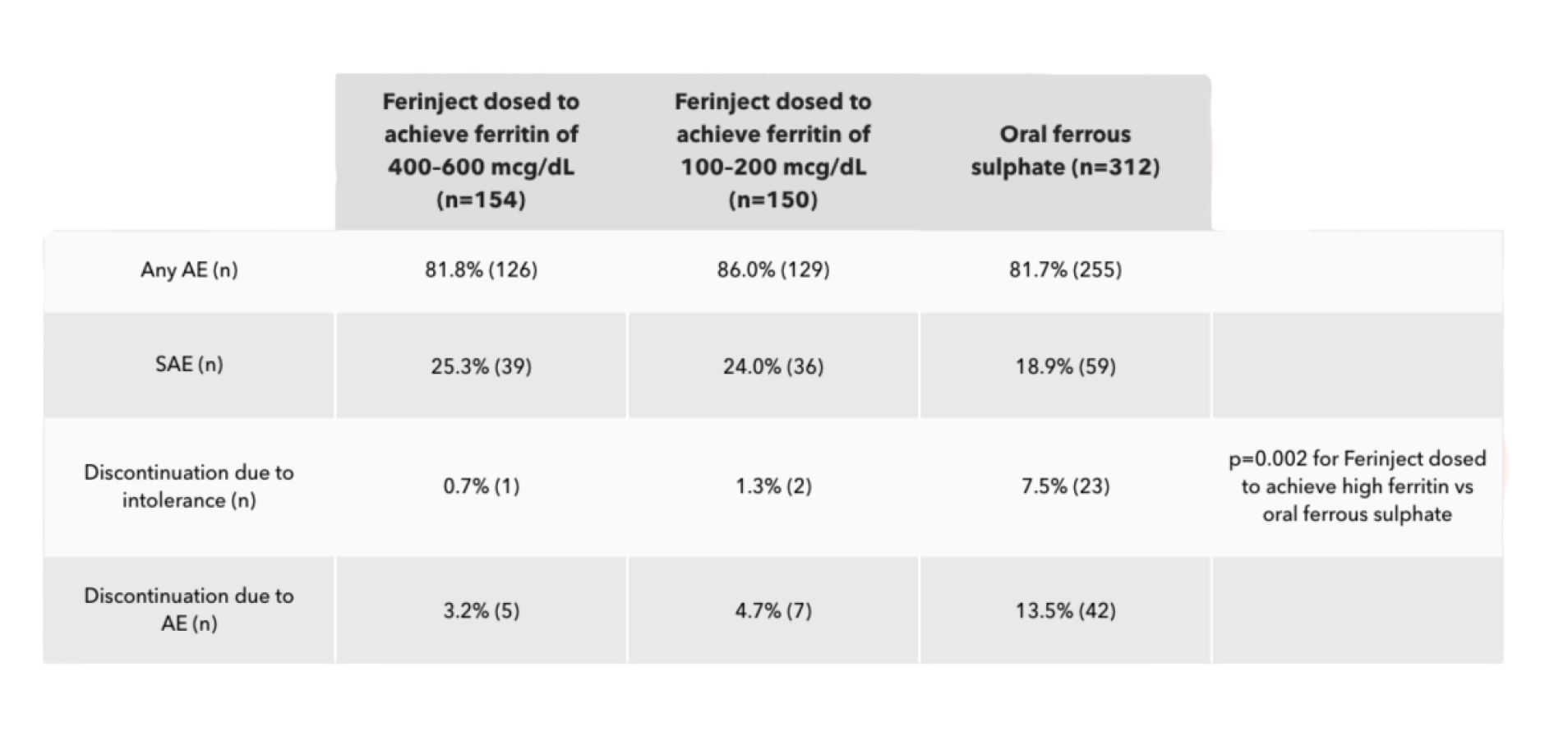
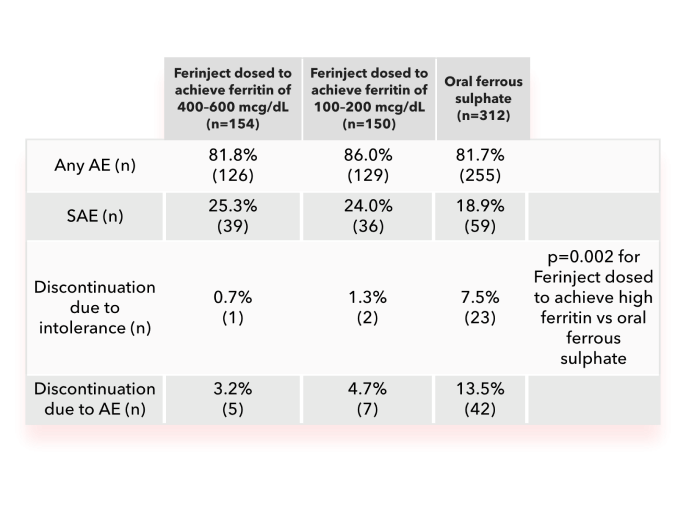
- In the Ferinject groups, the most common AEs were peripheral oedema, hypertension, urinary tract infection and back pain
- In the oral ferrous sulphate group, the most common AEs were diarrhoea, constipation, hypertension and peripheral oedema
- None of the SAEs in the Ferinject groups and one (0.3%) in the oral ferrous sulphate group were considered treatment-related
Ferinject has a well-characterised tolerability profile:1

Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions are immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration

If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
AE, adverse event; ADR, adverse drug reaction; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; FCM, ferric carboxymaltose; Hb, haemoglobin; HR, hazard ratio; IDA, iron deficiency anaemia; IV, intravenous; ND-CKD, non-dialysis chronic kidney disease; MDRD, Modification of Diet in Renal Disease; SAE, serious adverse event.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- Macdougall IC, Bock AH et al. Nephrol Dial Transplant 2014;29(11):2075–2084 (+ suppl).
Additional
DE-FCM-2100195
Efficacy and Safety
FIND-CKD: Ferinject treatment to achieve high ferritin levels in patients with chronic kidney disease and iron deficiency1,2
Key learning points
- Achieving higher ferritin levels with Ferinject in patients with iron deficiency and non-dialysis chronic kidney disease (ND-CKD) may lead to more effective IDA management vs achieving low ferritin levels with oral ferrous sulphate or intravenous (IV) Ferinject2
- Treating IDA with Ferinject to achieve high ferritin levels was not associated with an increased adverse event (AE) rate vs achieving low ferritin levels with Ferinject2
Results from the FIND-CKD study: treating iron deficiency to achieve high ferritin levels with Ferinject in patients with ND-CKD and IDA
FIND-CKD was a 56-week open-label, multi-centre, randomised-controlled, 3-arm study in 626 patients with CKD Stage 3–5 not yet on dialysis and with IDA. In this study, there were two Ferinject arms:
- Ferinject dosed to achieve ferritin levels of 400-600 mcg/L
- Ferinject dosed to achieve ferritin levels of 100-200 mcg/L; the comparator arm was oral ferrous sulphate dosed to maintain ferritin between 100 and 200 mcg/L
The primary endpoint was time to initiation of other anaemia management (erythropoiesis-stimulating agent (ESA), other iron therapy or blood transfusion) or occurrence of a haemoglobin (Hb) trigger of two consecutive values <10 g/dL during Weeks 8-52.2
FIND-CKD study design2

Adapted from Macdougall IC et al, 2014.2
*Patients ≤66 kg: 500 mg iron on Days 0 and 7.2
†Patients ≤66 kg: 500 mg iron on day of visit and 500 mg iron 1 week later.2
‡No administration if transferrin saturation level ≥40%.2
§Oral ferrous sulphate was withheld if serum ferritin was >200 mcg/L and restarted if/when serum ferritin was <100 mcg/L.2 The last dose of Ferinject was administered at Week 48, and the last dose of ferrous sulphate was administered at Week 52.2
In patients with IDA and ND-CKD, achieving high ferritin levels (400-600 mcg/L) with Ferinject significantly reduced and/or delayed the need for alternative anaemia management or the occurrence of an Hb trigger compared with achieving ferritin levels of 100-200 mcg/L with oral ferrous sulphate2
36/153 patients (23.5%) Ferinject vs 98/308 patients (31.8%) oral ferrous sulphate; HR: 0.65 (95% Cl: 0.44-0.95); p=0.026
The difference in the primary endpoint of time to initiation of an alternative treatment for anaemia or occurrence of a Hb trigger between Ferinject dosed to achieve high ferritin (400-600 mcg/L) and Ferinject dosed to achieve lower ferritin (100-200 mcg/L) was not statistically significant2
36/153 patients (23.5%) Ferinject high ferritin vs 49/153 patients (32.2%) Ferinject low ferritin; HR: 0.68 (95% Cl: 0.45-1.05); p=0.082

of patients with IDA and ND-CKD maintained a Hb level ≥10 g/dL or required no further anaemia treatment when treated with Ferinject dosed to achieve high ferritin levels of 400–600 mcg/L (n=117/153)2
The primary endpoint occurred in 36 patients (23.5%), 49 patients (32.2%) and 98 patients (31.8%) in the high-ferritin FCM, low-ferritin FCM and oral ferrous sulphate groups, respectively.2
In FIND-CKD, treating IDA with Ferinject dosed to achieve high ferritin levels was not associated with an increased AE rate vs achieving low ferritin levels with Ferinject2


- In the Ferinject groups, the most common AEs were peripheral oedema, hypertension, urinary tract infection and back pain
- In the oral ferrous sulphate group, the most common AEs were diarrhoea, constipation, hypertension and peripheral oedema
- None of the SAEs in the Ferinject groups and one (0.3%) in the oral ferrous sulphate group were considered treatment-related
Ferinject has a well-characterised tolerability profile:1

Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions are immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration

If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
AE, adverse event; ADR, adverse drug reaction; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; FCM, ferric carboxymaltose; Hb, haemoglobin; HR, hazard ratio; IDA, iron deficiency anaemia; IV, intravenous; ND-CKD, non-dialysis chronic kidney disease; MDRD, Modification of Diet in Renal Disease; SAE, serious adverse event.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- Macdougall IC, Bock AH et al. Nephrol Dial Transplant 2014;29(11):2075–2084 (+ suppl).
Additional
DE-FCM-2100195
Efficacy and Safety
Key learning points:
- Ferinject is a high-dose intravenous (IV) iron treatment for iron deficiency with a licence for use in adults and paediatric patients aged 1 year and older1
- The safety profile of Ferinject for children and adolescents aged 1 to 17 years is comparable with that of adults1
Ferinject is licensed for patients aged 1 year and older to efficiently deliver IV iron1
As of February 2023, Ferinject is approved for children and adolescent patients aged 1–13 years as well as adult and adolescent patients aged 14 years and older.1
The efficacy and safety of Ferinject has not been investigated in children below 1 year of age. Ferinject is therefore not recommended for use in children in this age group.1
The efficacy and safety profile of Ferinject in the paediatric population1
Paediatric studies were performed in children and adolescents as outlined below:1

Open-label, phase III study in children and adolescents aged 1-17 years with iron deficiency anaemia (IDA)

40 patients (median age 14.5 years) treated with two doses of 15 mg/kg body weight IV Ferinject at a 7-day interval (maximum single dose 750 mg)

39 patients (median age 14.0 years) treated with oral ferrous sulphate for 28 days
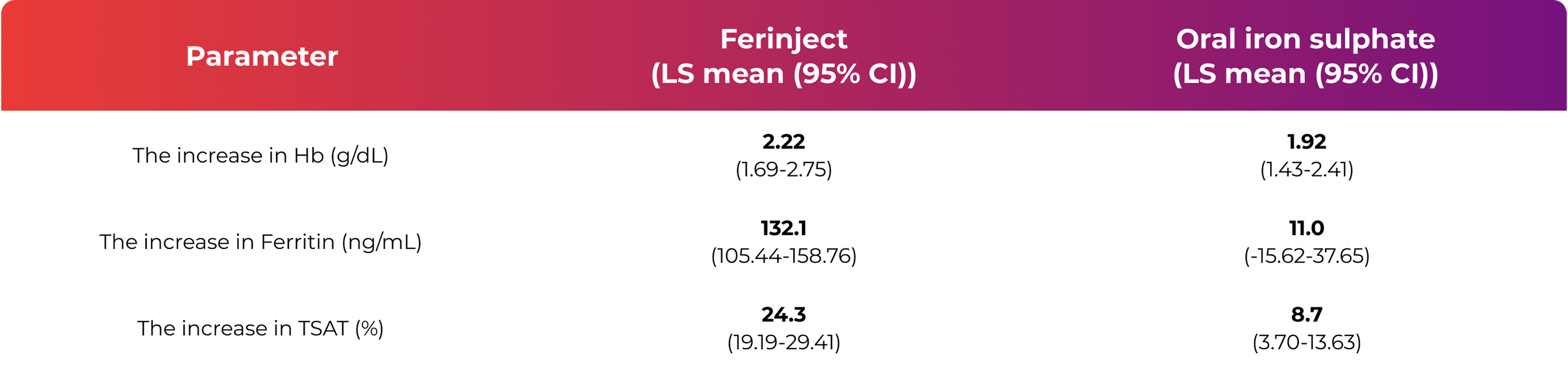
From baseline to Day 35, there was an increase in haemoglobin (Hb), ferritin and transferrin saturation (TSAT)1
- A similar increase in Hb was observed after both treatment with Ferinject and treatment with oral iron sulphate
- The increase in ferritin and TSAT, used as a measure for the replenishment of iron stores, was higher after Ferinject therapy compared to oral iron sulphate therapy
Tolerability of Ferinject in the paediatric population1
The safety profile for children and adolescents aged 1‑17 years is comparable to that of adults. 110 paediatric
patients received Ferinject in seven clinical studies. No serious adverse drug reactions (ADRs) were reported.
The reported non‑serious ADRs were:

hypophosphataemia
(n=5)
urticaria
(n=5)

injection/infusion site reactions
(n=4)

abdominal pain
(n=2)

flushing
(n=2)

headache
(n=2)

pyrexia
(n=2)
liver enzymes increased
(n=2)
rash
(n=2)
Constipation, gastritis, hypertension, pruritus and thirst were reported only once.
Refer to the Ferinject Summary of Product Characteristics for more information.
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
Download a leaflet for carers of children prescribed Ferinject
References & footnotes
Footnotes
ADR, adverse drug reaction; Cl, confidence interval; Hb, haemoglobin; IV, intravenous; LS, least squares; TSAT, transferrin saturation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk
Efficacy and Safety
Key learning points:
- Ferinject is a high-dose intravenous (IV) iron treatment for iron deficiency with a licence for use in adults and paediatric patients aged 1 year and older1
- The safety profile of Ferinject for children and adolescents aged 1 to 17 years is comparable with that of adults1
Ferinject is licensed for patients aged 1 year and older to efficiently deliver IV iron1
As of February 2023, Ferinject is approved for children and adolescent patients aged 1–13 years as well as adult and adolescent patients aged 14 years and older.1
The efficacy and safety of Ferinject has not been investigated in children below 1 year of age. Ferinject is therefore not recommended for use in children in this age group.1
The efficacy and safety profile of Ferinject in the paediatric population1
Paediatric studies were performed in children and adolescents as outlined below:1

Open-label, phase III study in children and adolescents aged 1-17 years with iron deficiency anaemia (IDA)

40 patients (median age 14.5 years) treated with two doses of 15 mg/kg body weight IV Ferinject at a 7-day interval (maximum single dose 750 mg)

39 patients (median age 14.0 years) treated with oral ferrous sulphate for 28 days
From baseline to Day 35, there was an increase in haemoglobin (Hb), ferritin and transferrin saturation (TSAT)1
- A similar increase in Hb was observed after both treatment with Ferinject and treatment with oral iron sulphate
- The increase in ferritin and TSAT, used as a measure for the replenishment of iron stores, was higher after Ferinject therapy compared to oral iron sulphate therapy

Tolerability of Ferinject in the paediatric population1
The safety profile for children and adolescents aged 1‑17 years is comparable to that of adults. 110 paediatric
patients received Ferinject in seven clinical studies. No serious adverse drug reactions (ADRs) were reported.
The reported non‑serious ADRs were:

hypophosphataemia
(n=5)

urticaria
(n=5)

injection/infusion site reactions
(n=4)

abdominal pain
(n=2)

flushing
(n=2)

headache
(n=2)

pyrexia
(n=2)

liver enzymes increased
(n=2)

rash
(n=2)
Constipation, gastritis, hypertension, pruritus and thirst were reported only once.
Refer to the Ferinject Summary of Product Characteristics for more information.
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
Download a leaflet for carers of children prescribed Ferinject
References & footnotes
Footnotes
ADR, adverse drug reaction; Cl, confidence interval; Hb, haemoglobin; IV, intravenous; LS, least squares; TSAT, transferrin saturation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk
Hypophosphataemia
Hypophosphataemia and hypophosphataemic osteomalacia are potential adverse drug reactions of Ferinject treatment for iron deficiency1

Key learning points
- Hypophosphataemia (HPP) is a common (≥1/100 to <1/10) adverse drug reaction (ADR) after treatment with Ferinject1–3
- A post-hoc analysis with pooled data suggested that a serum phosphate reduction may be observed after Ferinject administration, with the lowest reading at 2 weeks. Serum phosphate usually returned to baseline 8–12 weeks post‑administration2
- Certain groups of patients are at higher risk of developing symptomatic HPP after Ferinject administration than others2,3
HPP after Ferinject administration is common (≥1/100 to <1/10) and usually transient.1–3
Serum phosphate decreased after Ferinject administration with the lowest reading at 2 weeks and returned to baseline within 8–12 weeks2

Change in serum phosphate levels following Ferinject administration was assessed in a post-hoc pooled analysis of 45 CSL Vifor or partner‐sponsored clinical trials, in which 6,879 patients were treated with Ferinject and had serum phosphate measurements.2
At baseline, 90.4% of patients were anaemic, mean serum ferritin was 43.5 mcg/L, mean TSAT was 14.3% and 98.2% of patients had normal serum phosphate values ≥2.5 mg/dL. Mean cumulative dose received was 1,315 mg (min: 12.5 mg; max: 6,500 mg). Mean (SD) number of administrations was 2.2 (2.02).2
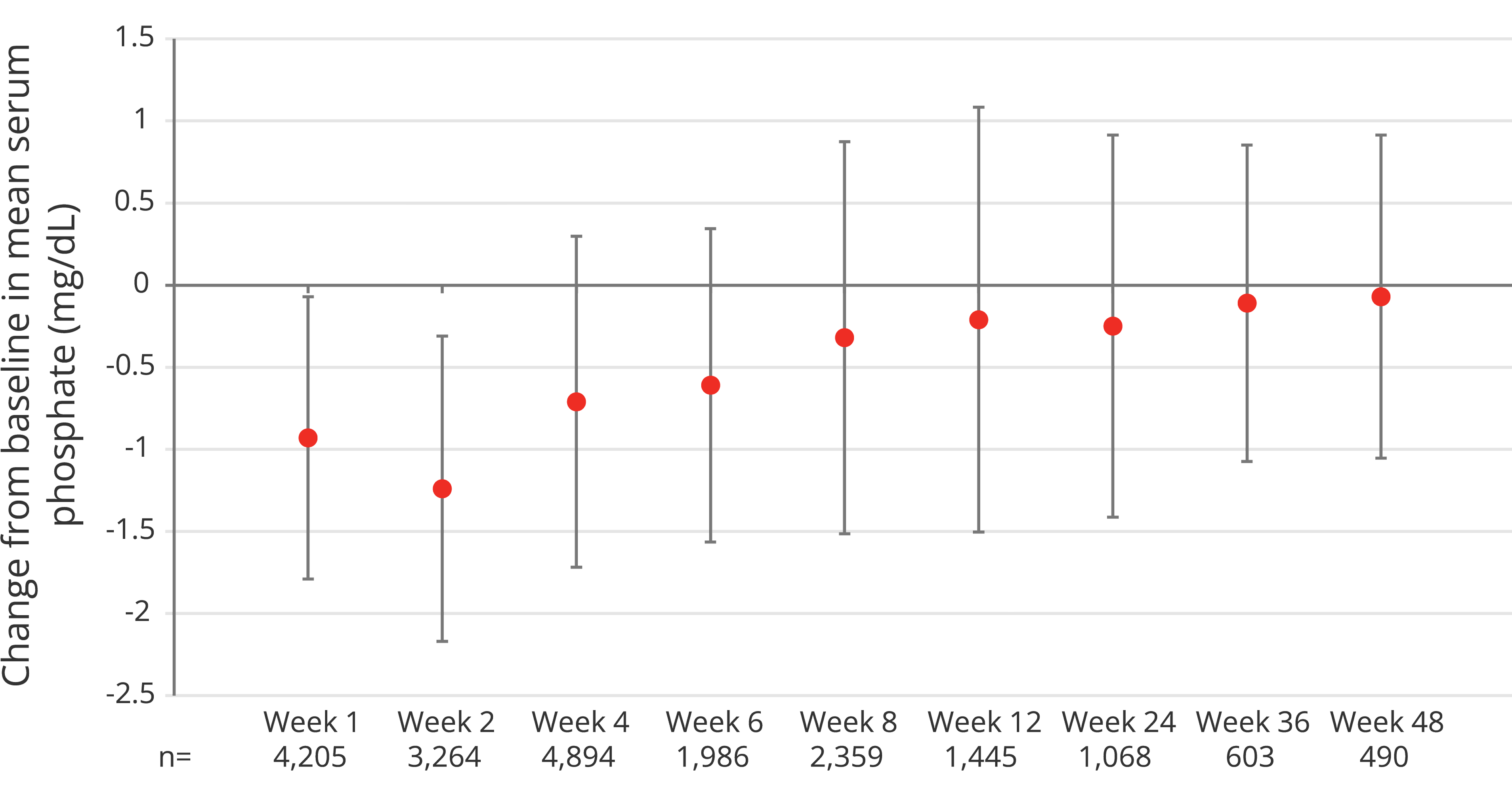
Adapted from Rosano G et al, 20202
A 1.24 mg/dL reduction in mean serum phosphate levels from 3.90 mg/dL at baseline to 2.69 mg/dL was observed in the first 2 weeks following treatment with Ferinject, before recovering to 3.22 mg/dL at Week 4 and 3.62 mg/dL at Week 8. No change in serum phosphate levels was observed in control or placebo groups.2
- Among all subjects receiving Ferinject therapy (N=6,879), 41.4% (n=2,847) reached a serum phosphate nadir value of <2.5 mg/dL, and 0.7% (n=49) reached a nadir value of <1 mg/dL (indicating severe HPP)2
- Multiple administrations of Ferinject at higher doses were identified to be risk factors for severe HPP2
Symptomatic HPP leading to osteomalacia and fractures has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain1
Certain groups of patients are at a higher risk of developing symptomatic HPP after Ferinject administration than others2,3

Risk factors for developing symptomatic HPP after Ferinject administration include:2,3
Vitamin D deficiency
Calcium malabsorption

Severe iron deficiency*

Multiple Ferinject doses in patients
requiring repeated high dosing

Secondary hyperparathyroidism
in high-risk populations†
*Low ferritin and ongoing blood loss mainly in patients with gastrointestinal or gynaecological conditions.3
†High-risk populations include Black/African race and high body mass index.2
Serum phosphate should be monitored in patients who receive long‑term treatment or multiple administrations at higher doses, and in those with existing risk factors for HPP1
In case of persisting HPP, treatment with Ferinject should be re‑evaluated.1
Mechanism of HPP following Ferinject administration:3

HPP after treatment with Ferinject is associated with increased urinary phosphate excretion. This is mediated by the phosphaturic hormone fibroblast growth factor 23 (FGF23), which is increased 3–6 fold after Ferinject infusion.
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Abbreviations
ADR, adverse drug reaction; FGF23, fibroblast growth factor 23; HPP, hypophosphataemia; SD, standard deviation; TSAT, transferrin saturation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk
- Rosano G, et al. J Clin Med 2020;9(11):3587.
- Schaefer B, et al. Bone 2022;154:116202.
Hypophosphataemia
Hypophosphataemia and hypophosphataemic osteomalacia are potential adverse drug reactions of Ferinject treatment for iron deficiency1

Key learning points
- Hypophosphataemia (HPP) is a common (≥1/100 to <1/10) adverse drug reaction (ADR) after treatment with Ferinject1–3
- A post-hoc analysis with pooled data suggested that a serum phosphate reduction may be observed after Ferinject administration, with the lowest reading at 2 weeks. Serum phosphate usually returned to baseline 8–12 weeks post‑administration2
- Certain groups of patients are at higher risk of developing symptomatic HPP after Ferinject administration than others2,3
HPP after Ferinject administration is common (≥1/100 to <1/10) and usually transient.1–3
Serum phosphate decreased after Ferinject administration with the lowest reading at 2 weeks and returned to baseline within 8–12 weeks2

Change in serum phosphate levels following Ferinject administration was assessed in a post-hoc pooled analysis of 45 CSL Vifor or partner‐sponsored clinical trials, in which 6,879 patients were treated with Ferinject and had serum phosphate measurements.2
At baseline, 90.4% of patients were anaemic, mean serum ferritin was 43.5 mcg/L, mean TSAT was 14.3% and 98.2% of patients had normal serum phosphate values ≥2.5 mg/dL. Mean cumulative dose received was 1,315 mg (min: 12.5 mg; max: 6,500 mg). Mean (SD) number of administrations was 2.2 (2.02).2

Adapted from Rosano G et al, 20202
A 1.24 mg/dL reduction in mean serum phosphate levels from 3.90 mg/dL at baseline to 2.69 mg/dL was observed in the first 2 weeks following treatment with Ferinject, before recovering to 3.22 mg/dL at Week 4 and 3.62 mg/dL at Week 8. No change in serum phosphate levels was observed in control or placebo groups.2
- Among all subjects receiving Ferinject therapy (N=6,879), 41.4% (n=2,847) reached a serum phosphate nadir value of <2.5 mg/dL, and 0.7% (n=49) reached a nadir value of <1 mg/dL (indicating severe HPP)2
- Multiple administrations of Ferinject at higher doses were identified to be risk factors for severe HPP2
Symptomatic HPP leading to osteomalacia and fractures has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain1
Certain groups of patients are at a higher risk of developing symptomatic HPP after Ferinject administration than others2,3

Risk factors for developing symptomatic HPP after Ferinject administration include:2,3

Vitamin D deficiency

Calcium malabsorption

Severe iron deficiency*

Multiple Ferinject doses in patients
requiring repeated high dosing

Secondary hyperparathyroidism
in high-risk populations†
*Low ferritin and ongoing blood loss mainly in patients with gastrointestinal or gynaecological conditions.3
†High-risk populations include Black/African race and high body mass index.2
Serum phosphate should be monitored in patients who receive long‑term treatment or multiple administrations at higher doses, and in those with existing risk factors for HPP1
In case of persisting HPP, treatment with Ferinject should be re‑evaluated.1
Mechanism of HPP following Ferinject administration:3

HPP after treatment with Ferinject is associated with increased urinary phosphate excretion. This is mediated by the phosphaturic hormone fibroblast growth factor 23 (FGF23), which is increased 3–6 fold after Ferinject infusion.
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Abbreviations
ADR, adverse drug reaction; FGF23, fibroblast growth factor 23; HPP, hypophosphataemia; SD, standard deviation; TSAT, transferrin saturation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk
- Rosano G, et al. J Clin Med 2020;9(11):3587.
- Schaefer B, et al. Bone 2022;154:116202.
Iron Deficiency Anaemia in Oncology
Iron deficiency in patients receiving treatment for cancer
Key learning points:
- Anaemia is common in patients receiving cancer treatment2,3
- The symptomatic burden of anaemia in patients with cancer has a detrimental effect on quality of life4
- Iron deficiency anaemia is a modifiable comorbidity in patients with cancer and it can be treated2
Anaemia is a common comorbidity in patients with cancer2,3
In the European Cancer Anaemia Survey (ECAS):

14,912
patients with cancer were evaluated for up to 6 months3 (15,367 enrolled)

39.3%
of patients presented with anaemia3*
(n=5,706/14,520)

67%
of patients had anaemia* at some point during the study3 (n=9,131/13,628)

39.3%
of patients with anaemia had a Hb <10 g/dL3
(n=5,860/14,912)
Patients receiving chemotherapy had the highest incidence of anaemia vs patients who did not receive any treatment, patients receiving radiotherapy and patients receiving concomitant chemo-radiotherapy3
(62.7% (n=3,301/5,265) vs 41.9% (n=2,206/5,265)
and 19.5% (n=1,027/5,265), respectively)
The incidence of anaemia increased with subsequent chemotherapy cycles3
(19.5% (n=533/2,732) in first cycle vs 46.7%
(n=1,276/2,732) in fifth cycle)
*Anaemia was defined as Hb <12.0 g/dL.3
Burden of anaemia in cancer
Anaemia in cancer may have consequences for patient prognosis:
Tumour response5
In patients with anaemia, low Hb levels may impact chemotherapy response rate in breast cancer
Progression‑free survival (PFS)6
Anaemia was associated with poor PFS in patients with prostate cancer
Overall survival (OS)7
Post‑diagnosis Hb change may be a predictor of OS in patients with lung, breast, colorectal and liver cancer in patients with anaemia
Mortality8
There may be an overall estimated 65% increase in the risk of mortality in patients with cancer and anaemia vs those without anaemia
Anaemia in cancer is complex and multifactorial but functional iron deficiency is one of the main contributors9
Common causes of iron deficiency anaemia in patients with cancer:
ESMO guidelines for assessing iron status in patients with cancer1Absolute iron deficiency9
Total body iron depleted with low iron stores
Caused by:
- poor iron intake
- impaired absorption
- acute and chronic blood loss (e.g. surgery)
Functional iron deficiency9
Inability to access iron stores
Caused by:
- increased erythropoiesis (through blood loss or use of ESAs)
- systemic inflammation (which increases hepcidin levels)

Reduction of iron supply to all cells of the body

Iron deficiency or iron deficiency anaemia
Other causes of anaemia in cancer include:9
- vitamin B12 and folate deficiency
- chemotherapy-induced anaemia (bone marrow and renal toxicity, red cell haemolysis)
- tumour-induced anaemia (bone marrow metastasis)
Ferinject is only indicated for the treatment of anaemia caused by iron deficiency1
Multiple factors are associated with iron deficiency and anaemia in oncology10
A prospective study of 1,528 patients with cancer investigated the interdependencies between iron deficiency and various clinical parameters.10
Both iron deficiency and anaemia are more common at a more advanced stage of disease at diagnosis (p<0.001)
Iron deficiency and anaemia were most common in patients who had recently received their last anticancer therapy within 12 weeks vs those who had received it more than 12 weeks from baseline (p<0.001)
Patients with persistent or progressive disease were most likely to have iron deficiency or anaemia vs patients in complete remission (p<0.001)
Both iron deficiency and anaemia significantly correlated with poor ECOG performance status (p=0.005 and p=0.001, respectively)
The symptomatic burden of anaemia in patients with cancer has a detrimental effect on quality of life4
Anaemia is associated with symptoms of fatigue, lethargy, dyspnoea, loss of appetite and inability to concentrate, which may have a negative impact on quality of life.4
In a study of patients with cancer and anaemia (n=179), there were significant positive relationships between Hb levels and...4

6 of 6
…domains of the Functional Assessment of Cancer Therapy – Anaemia†

6 of 8
…of the SF-36 domains†
†FACT‑G, fatigue, non‑fatigue, anaemia, FACT‑F and FACT‑An.4
‡Physical function, role physical, social functioning, mental health, energy/vitality, health perception. Differences in pain and role mental were not significant.4
Iron deficiency management in patients with cancer
- Early anaemia management may help patients maintain Hb target levels during chemotherapy3
- Iron deficiency in anaemia is a modifiable comorbidity in patients with cancer and it can be treated2
- Raising Hb level may improve the quality of life of patients with cancer and anaemia4
Learn more about the treatment of iron deficiency and iron deficiency anaemia in patients with cancer
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
ADR, adverse drug reaction; ECAS, European Cancer Anaemia Survey; ECOG, Eastern Cooperative Oncology Group; ESA, erythropoiesis-stimulating agents; ESMO, European Society for Medical Oncology; FACT, Functional Assessment of Cancer Therapy; Hb, haemoglobin; OS, overall survival; PFS, progression free survival; SF-36, short form-36; TSAT, transferrin saturation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk.
- Aapro, et al. Ann Oncol 2018;29:iv96–iv110.
- Ludwig H, et al. Eur J Cancer 2004;40:2293–2306.
- Lind M, et al. Br J Cancer 2002;86:1243–1249.
- Bottini A, et al. Br J Cancer 2003;89:977–982.
- Dai D, et al. Am J Transl Res 2018;10:3877–3886.
- Wan S, et al. BMC Cancer 2013;13:340.
- Caro J J, et al. Cancer 2001;91:2214–2221.
- Busti F, et al. Pharmaceuticals (Basel) 2018;11:94.
- Ludwig H, et al. Ann Oncol 2013;24:1886–1892.
Why Ferinject?
Why Choose Ferinject for your patients with iron deficiency (ID)?
Key learning points
- Ferinject’s heritage includes over 29 million patient-years of cumulative exposure and 15+ years of clinical use1,2
- Ferinject has been studied in over 9,000 patients across 44 randomised control trials (RCTs), creating an evidence base across a broad range of therapeutic areas3
- Ferinject is approved for use in both children and adolescents aged 1–13 years,* and adults and adolescents aged 14 years and older1
- Ferinject has a resilient and agile supply chain, built on decades of experience, giving confidence in access to Ferinject and continuity of patient care
*The efficacy and safety of Ferinject has not been investigated in children below 1 year of age. Ferinject is therefore not recommended for use in children in this age group.1 Ferinject has not been investigated in children aged 1–13 years with chronic kidney disease requiring haemodialysis and is therefore not recommended for use in this patient population.1
Ferinject: a legacy of trust in iron-based therapies

Approved in 87 countries across the world4

Safety data collected in over 9,000 patients, including >100 children and adolescents aged 1–17 years1

Since launch, Ferinject has had over 29 million patient‑years of cumulative exposure, with over 15 years of clinical use1,2
Ferinject’s trusted supply network
At CSL, our supply network is built on decades of experience in iron therapy and long-standing partnerships across a robust and reliable ecosystem. This trusted infrastructure ensures resilience, agility, and continuity, so our customers can depend on consistent access to high-quality iron treatments for their patients.
Our ability to respond quickly to changing demands is underpinned by both manual and automated packaging capabilities. This flexibility gives our customers confidence that supply will be consistent and timely. For Ferinject, a trusted supply network means more than operational excellence; it's a commitment to supporting our customers deliver high-quality patient care.
Ferinject: Backed by a robust evidence base across a range of therapeutic areas3
Ferinject has been studied in over 9,000 patients across 44 company- or partner-sponsored RCTs3

Cardiology
6 studies
Patients receiving Ferinject: n=1,154
Patients receiving
Ferinject: n=1,154
Total patients: N=2,152

Nephrology
5 studies
Patients receiving Ferinject: n=2,100
Patients receiving
Ferinject: n=2,100
Total patients: N=4,177
Oncology
3 studies
Patients receiving Ferinject: n=131
Patients receiving
Ferinject: n=131
Total patients: N=261
Mixed
10 studies
Patients receiving Ferinject: n=2,601
Patients receiving
Ferinject: n=2,601
Total patients: N=4,655

Gastroenterology
5 studies
Patients receiving Ferinject: n=546
Patients receiving
Ferinject: n=546
Total patients: N=1,149

Women’s health
9 studies
Patients receiving Ferinject: n=2,060
Patients receiving
Ferinject: n=2,060
Total patients: N=4,048

Neurology
5 studies
Patients receiving Ferinject: n=394
Patients receiving
Ferinject: n=394
Total patients: N=738

Paediatrics
1 study
Patients receiving Ferinject: n=40
Patients receiving
Ferinject: n=40
Total patients: N=79
Ferinject is an effective way to deliver up to 1,000 mg of intravenous (IV) iron to your patients1
Ferinject can be delivered by infusion or injection in just 15 minutes, which must be followed by a 30-minute observation period.1
The minimum administration time is 15 minutes for a 1,000 mg dose, with an observation time of 30 minutes post administration.1
Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration.1
For patients aged 1–13 years old, a single Ferinject administration should not exceed:1
- 15 mg iron/kg body weight
- 750 mg of iron (15 mL Ferinject)
The maximum recommended cumulative dose of Ferinject is 750 mg of iron (15 mL Ferinject) per week in patients aged 1–13 years. If the total iron need is higher, then the administration of an additional dose should be a minimum of 7 days apart from the first dose.1
For patients 14 years and older, a single Ferinject administration should not exceed:1
- 15 mg iron/kg body weight (for administration by IV injection) or 20 mg iron/kg body weight (for administration by IV infusion)
- 1,000 mg iron (20 mL Ferinject)
The maximum recommended cumulative dose of Ferinject is 1,000 mg iron (20 mL Ferinject) per week. If the total iron need is higher, then the administration of an additional dose should be a minimum of 7 days apart from the first dose.1
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
ADR, adverse drug reaction; Cl, confidence interval; Hb, haemoglobin; HPP, hypophosphataemia; ID, iron deficiency; IV, intravenous; LS, least squares; RCT, randomised controlled trial; TSAT, transferrin saturation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk
- Vifor Pharma UK Ltd. Data on File 158.
- Vifor Pharma UK Ltd. Data on File 150.
- CSL (2024). Ferinject® approved by Health Canada for the treatment of iron deficiency anemia in adult and pediatric patients and iron deficiency in adult patients with heart failure. Available at: newsroom.csl.com. Accessed October 2025.
Dosing and administration
Key learning points:
- Ferinject is licensed for use in patients aged 1-13 years, administered via the intravenous (IV) route and not exceeding 15 mL (750 mg of iron) per week1
- Ferinject can be administered in up to 15 minutes, which must be followed by at least a 30-minute observation period1
Dosing and administration in patients aged 1–13 years
Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is
immediately available and where full resuscitation facilities can be assured. The patient should be observed for
adverse effects during and for at least 30 minutes following each Ferinject administration, especially for signs
and symptoms of hypersensitivity reactions.1
The posology of Ferinject follows a stepwise approach:1
assessments
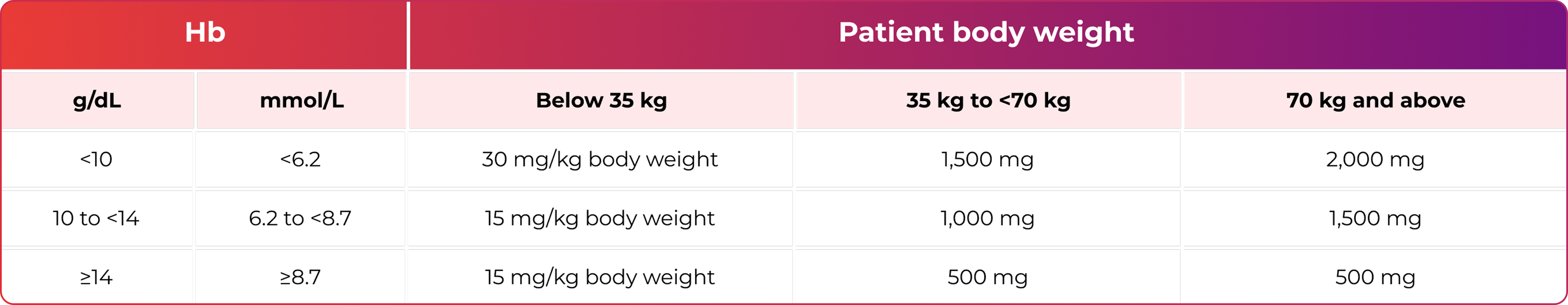
Two doses may be required to replenish the total iron need.1
For patients aged 1–13 years old, a single Ferinject administration should not exceed:1
- 15 mg iron/kg body weight
- 750 mg of iron (15 mL Ferinject)
The maximum recommended cumulative dose of Ferinject is 750 mg of iron (15 mL Ferinject) per week in patients aged 1–13 years. If the total iron need is higher, then the administration of an additional dose should be a minimum of 7 days apart from the first dose.1
For patients 14 years and older, a single Ferinject administration should not exceed:1
- 15 mg iron/kg body weight (for administration by IV injection) or 20 mg iron/kg body weight (for administration by IV infusion)
- 1,000 mg iron (20 mL Ferinject)
The maximum recommended cumulative dose of Ferinject is 1,000 mg iron (20 mL Ferinject) per week. If the total iron need is higher, then the administration of an additional dose should be a minimum of 7 days apart from the first dose.
Re-assessment should be performed by the clinician based on the individual patient's condition:1
- The Hb level should be re‑assessed no earlier than 4 weeks post final Ferinject administration to allow adequate time for erythropoiesis and iron utilisation.
- In the event the patient requires further iron repletion, the iron need should be recalculated (see Step 1).
Ferinject must be administered to paediatric patients only via the IV
route by:1
injection
OR

infusion
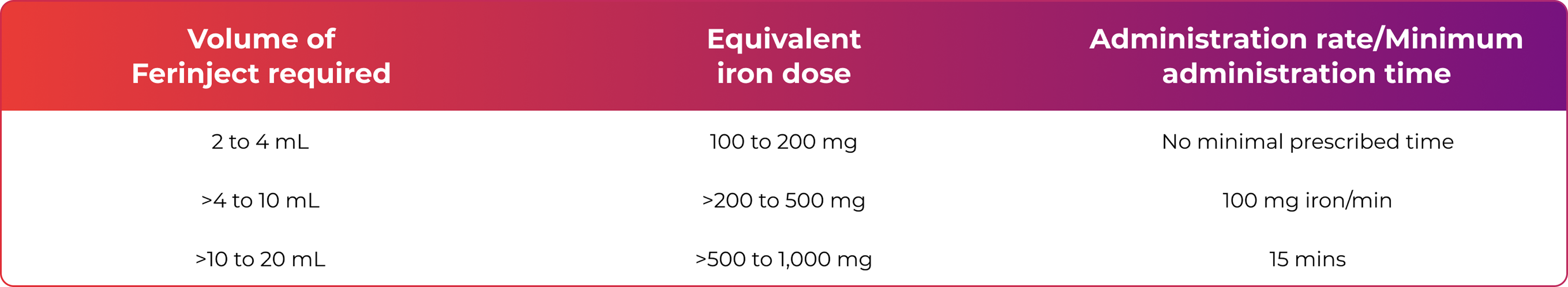
Ferinject must not be administered by the subcutaneous or intramuscular route.1
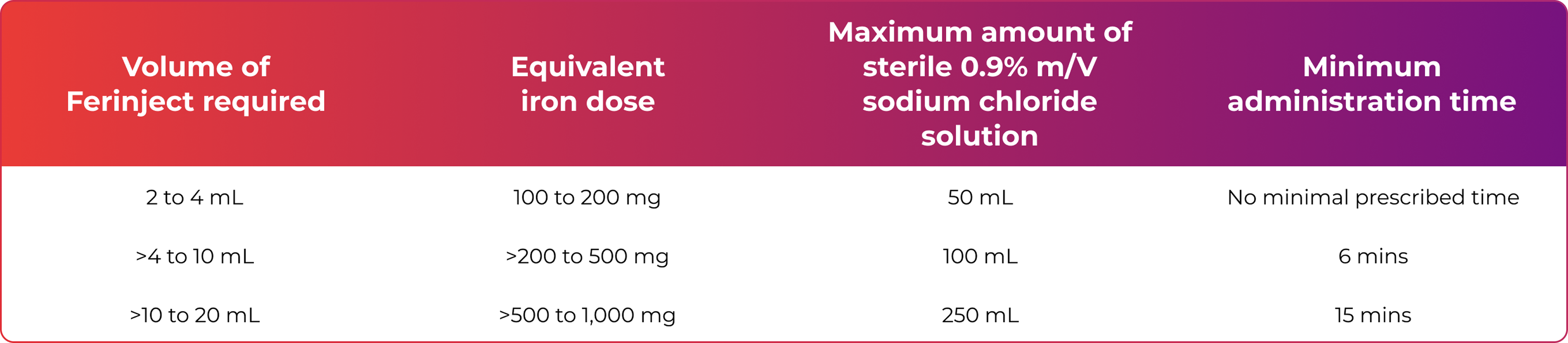
Ferinject must not be administered by the subcutaneous or intramuscular route.1
In children aged 1 to 13 years with chronic kidney disease requiring haemodialysis, the efficacy and safety profile of Ferinject has not been investigated. Ferinject is therefore not recommended for use in children aged 1 to 13 years with chronic kidney disease requiring haemodialysis.1
Please refer to the Summary of Product Characteristics for more information on administration rates and dilution plan.
Ferinject can be administered in up to 15 minutes, which must be followed by at least a 30-minute observation period.1
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
Download a summary of Ferinject dosing and administration for paediatric patients aged 1 to 13 years
References & footnotes
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk
Efficacy and Safety
Ferinject has been shown to improve clinical outcomes for your patients with gastrointestinal bleeding2,3
Key learning points
- Iron deficiency anaemia (IDA) may be caused by gastrointestinal (GI) bleeding4,5
- Iron deficiency (ID) is the main cause of anaemia in surgical patients and IDA can have a major impact on patient outcomes6–8
- Treatment of ID with Ferinject could benefit patients with GI bleeding9
IDA may be caused by GI bleeding.5,6 GI bleeding can be acute or chronic and can occur anywhere along the GI tract10
The causes of GI bleeding include:10
- peptic ulcer disease
- gastritis/duodenitis
- oesophageal varices
- Mallory-Weiss tear
- oesophagitis
- gastric cancer
- Dieulafoy's lesion
- gastric arteriovenous malformations
- portal gastropathy
- angiodysplasia
- jejunoileal diverticula
- Meckel's diverticulum
- neoplasms/lymphomas (benign and malignant)
- enteritis/Crohn's disease
- aortoduodenal fistula in patient with synthetic vascular graft
- diverticular disease
- arteriovenous malformations
- colitis
- colonic neoplasms/post-polypectomy bleeding
- anorectal causes
- colonic tuberculosis
ID is the main cause of anaemia in surgical patients6,7
Associative studies in patients undergoing surgery have shown that those with anaemia versus non-anaemeic patients may have:
Increased likelihood of blood transfusion, which in turn can:7
- be associated with increased mortality6
- delay hospital discharge/increase length of stay6
- Correcting IDA before surgery can reduce red blood cell (RBC) transfusion requirements3
- IDA must be monitored and managed in all incidences of GI bleeding2
Managing IDA in patients with GI bleeding
1. Diagnosis of IDA in patients with GI bleeding
The PRODIGGEST guideline recommends reviewing haemoglobin (Hb) and iron-parameters (ferritin, TSAT) in all patients with GI bleeding2
Diagnosis parameter thresholds:
ANAEMIA11
♀ ︎Hb <12 g/dL
♂ Hb <13 g/dL
IRON DEFICIENCY2
Ferritin: <30–100 mcg/L*
Transferrin: >300–350 mg/dL
TSAT: <20%
MCV: <81 fl
MCH: <28 pg
RDW: >15%
*<100 mcg/L may be indicative of iron deficiency in the presence of inflammation.
2. IDA treatment options for patients with GI bleeding
Treatment of IDA with iron replacement therapy should be considered in patients with GI bleeding. When severe anaemia is present (Hb <70 g/L), blood transfusion should br considered.2
IDA treatment goals in GI bleeding are:2
Haemodynamic stabilisation
RBC transfusion
- RBC transfusions may be required in haemodynamically unstable patients primarily due to acute GI bleeding12
- Implementing a restrictive RBC transfusion strategy significantly improves outcomes in patients with acute upper GI bleeding compared with a liberal RBC transfusion strategy13
- A liberal transfusion strategy is associated with increased mortality and unfavourable outcomes, possibly due to coagulation abnormalities and clot rupture14
Iron parameter normalisation (Hb and ferritin levels)
Iron therapy
Identify the most appropriate iron therapy for your patient

Intravenous (IV) iron is indicated when oral iron preparations are ineffective, oral iron preparations cannot be used or there is a clinical need to deliver iron rapidly1
ID and IDA Screening and Diagnostic Guidelines
Screening
- Establish anaemia is IDA by actively seeking the presence of ID
Screening parameters
- Serum ferritin (SF): Most useful marker for IDA
- Use other tests (e.g. transferrin saturation) if false-normal suspected
Diagnostic thresholds
- Anaemia: Hb below the lower limit of normal for the relevant population and laboratory
- ID: SF <45 µg/L suggestive of ID
Screening
- Inflammatory bowl disease (IBD) patients should be regularly assessed for anaemia. About two-thirds of such patients have anaemia at diagnosis.
- For remission or mild disease: Should be performed every 6 to 12 months
- In patients with active disease: Should be performed at least every 3 months
Screening parameters
- Complete blood count, SF and c‑reactive protein should be used
Diagnostic thresholds
- Anaemia: ECCO aligns with the WHO anaemia definition for defining anaemia in IBD Hb <13 g/dL in men and Hb <12 g/dL in women
- ID

“As SF is an acute phase protein, apparently normal levels may occur with iron deficiency in the context of an inflammatory disease process. A SF cut-off of 45 µg/L has been suggested as providing the optimal trade-off between sensitivity and specificity for iron deficiency in practice.”15
Ferinject efficacy
The efficacy of Ferinject was compared with that of oral ferrous sulphate in a prospective, randomised study in patients with anaemia secondary to non-variceal GI bleeding.9
Ferinject treatment resulted in:9
- rapid normalisation of mean Hb levels at 3 weeks after hospital discharge (85.7% of patients vs 45.2% of patients treated with ferrous sulphate; n=24/28 patients vs n=14/31 patients; p=0.001)
- complete response at the end of the study in 100% of patients (vs 61.3% of patient treated with ferrous sulphate; n=29/29 vs n=19/31; p=0.001)
- numerical decrease in the percentage of patients with mobility problems and pain/discomfort compared with an increase in the ferrous sulphate group (percentage of patients reporting mobility problems at Day 42: 8% in the Ferinject group (n=1/14) vs 20% in the ferrous sulphate group (n=4/19); percentage of patients reporting pain/discomfort at Day 42: 15% in the Ferinject group (n=2/14) vs 27% in the ferrous sulphate group (n=5/19); p=0.02)
Partial response rates over the duration of the study9*
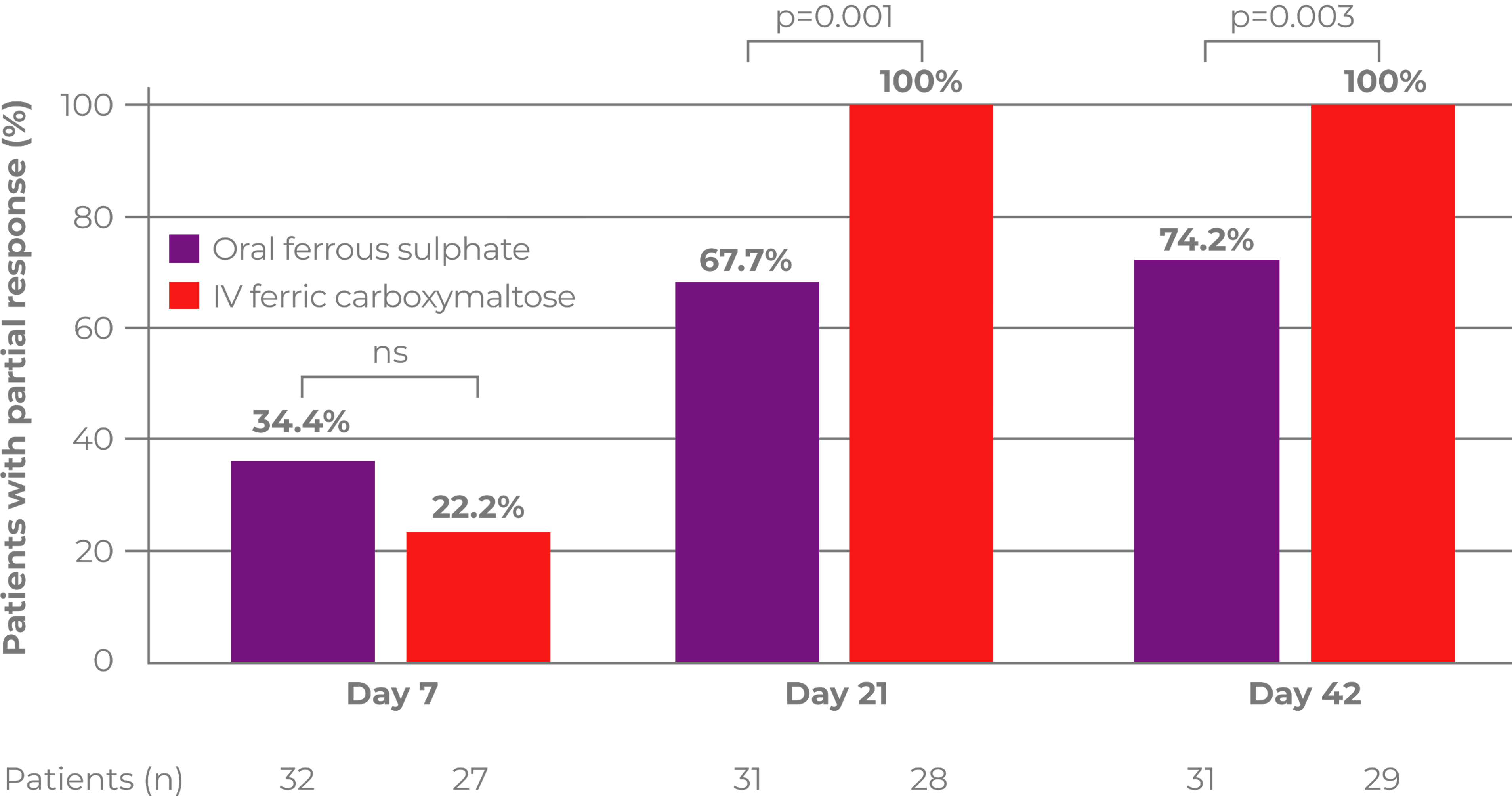
Adapted from Ferrer-Barcelo L et al, 20199
*Primary outcome was complete response after 6 weeks; complete response = Hb levels ≥12 g/dL or ≥13 g/dL in women and men, respectively, at Day 42.
Results of quality of life questionnaire (EQ‐5D‐3L)
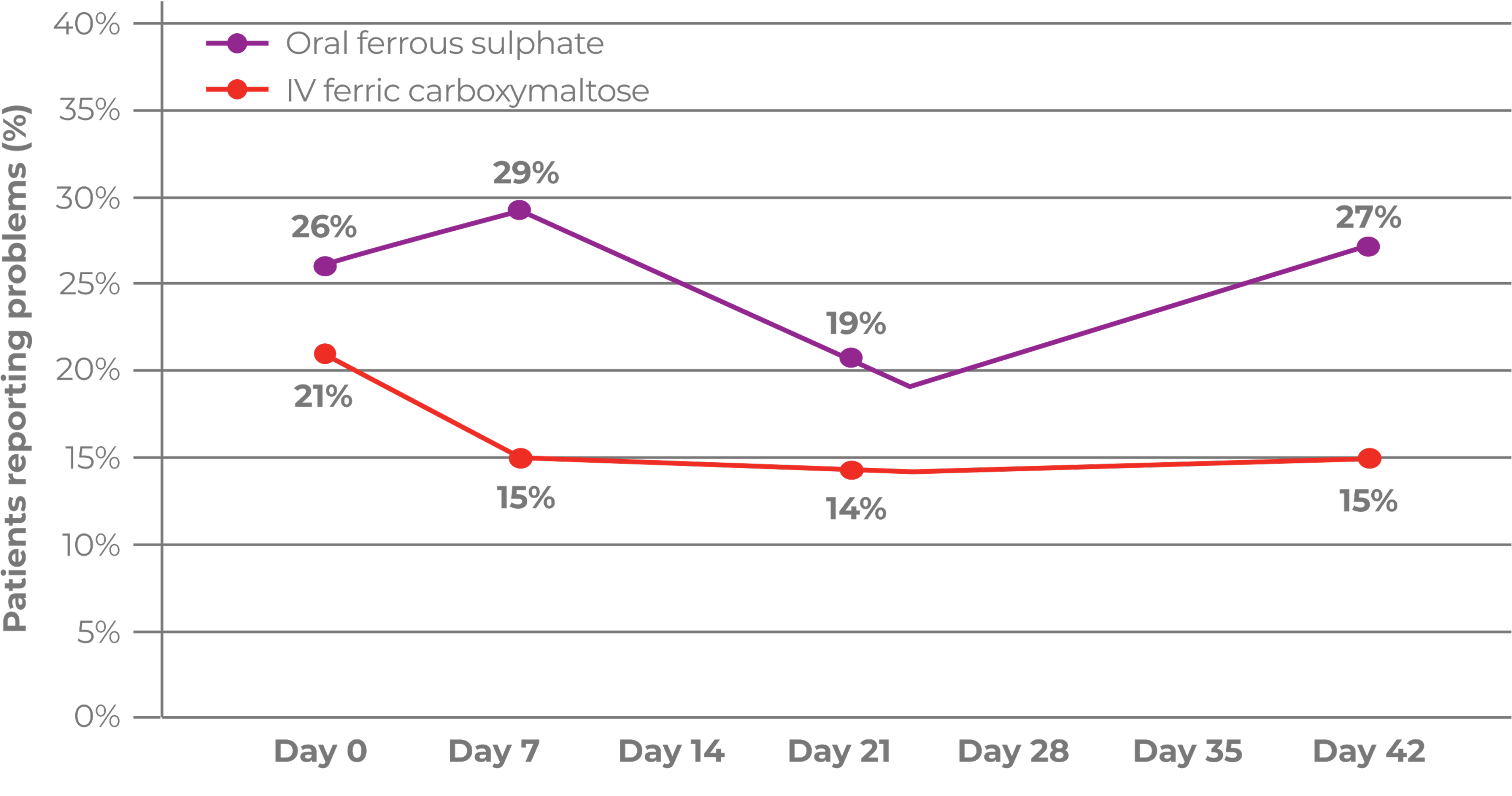
Adapted from Ferrer-Barcelo L et al, 20199
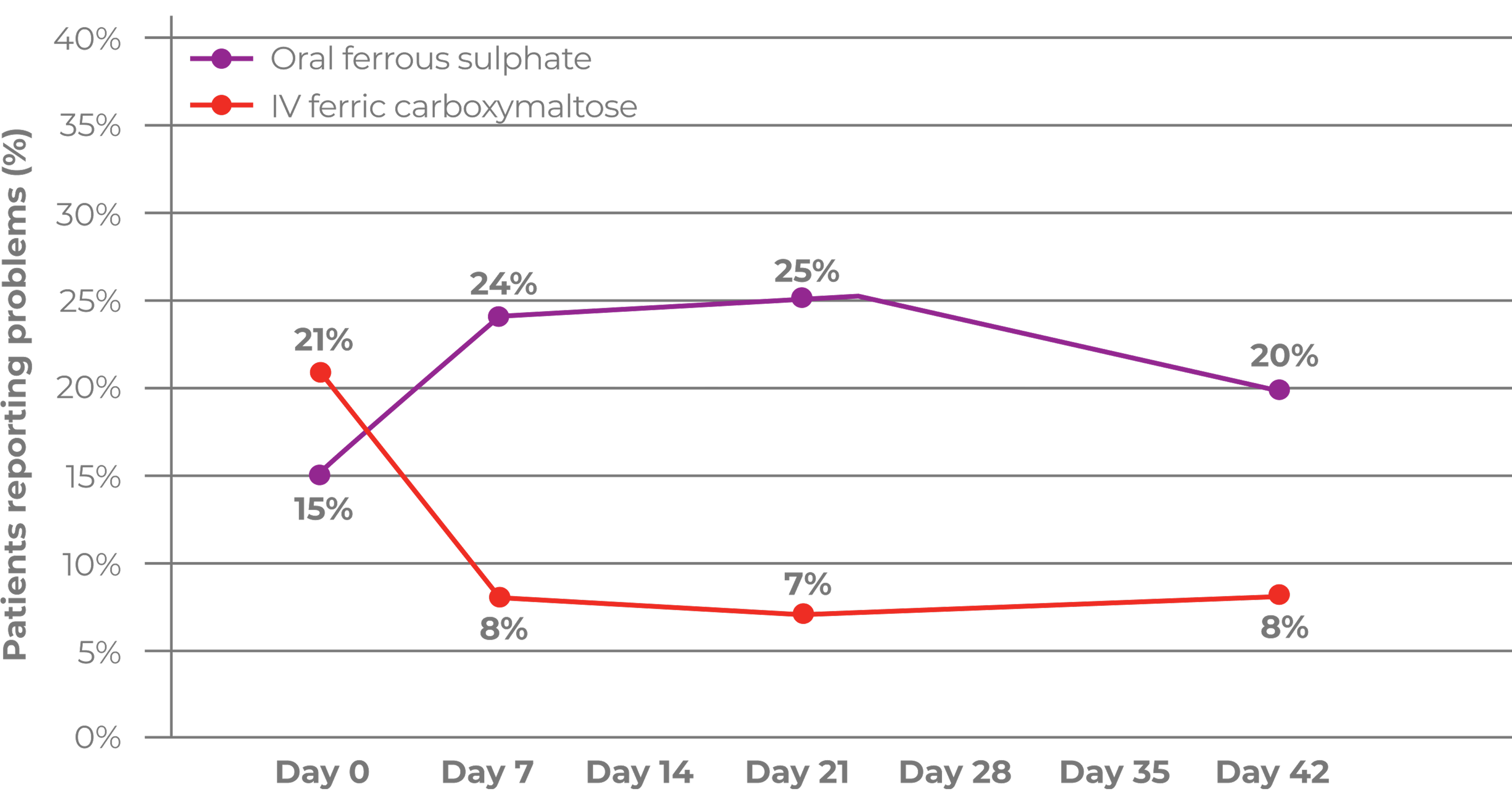
Adapted from Ferrer-Barcelo L et al, 20199
The EuroQoL 5 Dimensions (EQ‐5D‐3L) questionnaire was conducted face to face or via the telephone and assessed mobility, self-care, usual activities, anxiety/depression, and pain/discomfort. Higher percentages correspond to more patients with problems.9
The differences in pain/discomfort and mobility between the groups at day 42 were not significant.9
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
ADR, adverse drug reaction; BSG, British Society of Gastroenterology; CKD, chronic kidney disease; ECCO, European Crohn's and Colitis Organisation, EQ-5D-3L, EuroQoL 5 Dimensions; GI, gastrointestinal; Hb, haemoglobin; HPP, hypophosphataemia; IBD, inflammatory bowel disease; ID, iron deficiency; IDA, iron deficiency anaemia, IV, intravenous; MCH, mean corpuscular haemoglobin; MCV, mean corpuscular volume; ns, non-significant; PRODIGGEST, hospital PROtocols to improve interDIsciplinary manaGEment of gaSTrointestinal diseases in hospital settings; RDW, red cell distribution width; RBC, red blood cell; SD, standard deviation; SF, serum ferritin; TSAT, transferrin saturation; WHO, World Health Organisation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk
- PRODIGGEST (2017). Healthcare PROtocols to improve interDIsciplinary manaGEment of gaSTrointestinal diseases in hospital settings. Available at: https://www.aegastro.es/documents/prodiggest/Prodiggest-Management-of-anaemia-andiron-deficiency-in-gastrointestinal-bleeding.pdf. Accessed September 2025.
- Quinn EM, et al. Int J Surg 2017;38:1–8.
- Kaitha S, et al. World J Gastrointest Pathophysiol 2015;6(3):62–72.
- Gomollón F, Gisbert JP. World J Gastroenterol 2009;15(37):4659–4665.
- Khanbhal M, et al. ISRN Hematol 2014;2014:547914.
- Beattie WS, et al. Anesthesiology 2009;110(3):574–581.
- Tibi P, et al. Ann Thorac Surg 2021;112(3):981–1004.
- Ferrer-Barcelo L, et al. Aliment Pharamcol Ther 2019;50(3):258–268.
- Manning-Dimmitt LL, et al. Am Fam Physician 2005;71(7):1339–1346.
- World Health Organisation (WHO). Worldwide prevalence of anaemia 1993–2005. WHO Global Database on Anaemia. Available at: apps.who.int. Accessed September 2025.
- Bayraktar UD, Bayraktar S. World J Gastroenterol 2010;16(22):2720–2725.
- Villanueva C, et al. N Engl J Med 2013;368(1):11–21.
- Chen YC, et al. Clin Transl Gastroenterol 2018;9(3):138.
- Snook J, et al. Gut 2021;70:2030–2051.
- Diagnass AU, et al. J Crohns Colitis 2015:211–222.
Efficacy and Safety
Ferinject has been shown to improve clinical outcomes for your patients with gastrointestinal bleeding2,3
Key learning points
- Iron deficiency anaemia (IDA) may be caused by gastrointestinal (GI) bleeding4,5
- Iron deficiency (ID) is the main cause of anaemia in surgical patients and IDA can have a major impact on patient outcomes6–8
- Treatment of ID with Ferinject could benefit patients with GI bleeding9
IDA may be caused by GI bleeding.5,6 GI bleeding can be acute or chronic and can occur anywhere along the GI tract10
The causes of GI bleeding include:10
- peptic ulcer disease
- gastritis/duodenitis
- oesophageal varices
- Mallory-Weiss tear
- oesophagitis
- gastric cancer
- Dieulafoy's lesion
- gastric arteriovenous malformations
- portal gastropathy
- angiodysplasia
- jejunoileal diverticula
- Meckel's diverticulum
- neoplasms/lymphomas (benign and malignant)
- enteritis/Crohn's disease
- aortoduodenal fistula in patient with synthetic vascular graft
- diverticular disease
- arteriovenous malformations
- colitis
- colonic neoplasms/post-polypectomy bleeding
- anorectal causes
- colonic tuberculosis
ID is the main cause of anaemia in surgical patients6,7
Associative studies in patients undergoing surgery have shown that those with anaemia versus non-anaemeic patients may have:
Increased likelihood of blood transfusion, which in turn can:7
- be associated with increased mortality6
- delay hospital discharge/increase length of stay6

- Correcting IDA before surgery can reduce red blood cell (RBC) transfusion requirements3
- IDA must be monitored and managed in all incidences of GI bleeding2

Managing IDA in patients with GI bleeding
1. Diagnosis of IDA in patients with GI bleeding
The PRODIGGEST guideline recommends reviewing haemoglobin (Hb) and iron-parameters (ferritin, TSAT) in all patients with GI bleeding2
Diagnosis parameter thresholds:
ANAEMIA11
♀ ︎Hb <12 g/dL
♂ Hb <13 g/dL
IRON DEFICIENCY2
Ferritin: <30–100 mcg/L*
Transferrin: >300–350 mg/dL
TSAT: <20%
MCV: <81 fl
MCH: <28 pg
RDW: >15%
*<100 mcg/L may be indicative of iron deficiency in the presence of inflammation.
2. IDA treatment options for patients with GI bleeding
Treatment of IDA with iron replacement therapy should be considered in patients with GI bleeding. When severe anaemia is present (Hb <70 g/L), blood transfusion should br considered.2
IDA treatment goals in GI bleeding are:2
Haemodynamic stabilisation
RBC transfusion
- RBC transfusions may be required in haemodynamically unstable patients primarily due to acute GI bleeding12
- Implementing a restrictive RBC transfusion strategy significantly improves outcomes in patients with acute upper GI bleeding compared with a liberal RBC transfusion strategy13
- A liberal transfusion strategy is associated with increased mortality and unfavourable outcomes, possibly due to coagulation abnormalities and clot rupture14
Iron parameter normalisation (Hb and ferritin levels)
Iron therapy
Identify the most appropriate iron therapy for your patient

Intravenous (IV) iron is indicated when oral iron preparations are ineffective, oral iron preparations cannot be used or there is a clinical need to deliver iron rapidly1
ID and IDA Screening and Diagnostic Guidelines
Screening
- Establish anaemia is IDA by actively seeking the presence of ID
Screening parameters
- Serum ferritin (SF): Most useful marker for IDA
- Use other tests (e.g. transferrin saturation) if false-normal suspected
Diagnostic thresholds
- Anaemia: Hb below the lower limit of normal for the relevant population and laboratory
- ID: SF <45 µg/L suggestive of ID
Screening
- Inflammatory bowl disease (IBD) patients should be regularly assessed for anaemia. About two-thirds of such patients have anaemia at diagnosis.
- For remission or mild disease: Should be performed every 6 to 12 months
- In patients with active disease: Should be performed at least every 3 months
Screening parameters
- Complete blood count, SF and c‑reactive protein should be used
Diagnostic thresholds
- Anaemia: ECCO aligns with the WHO anaemia definition for defining anaemia in IBD Hb <13 g/dL in men and Hb <12 g/dL in women
- ID

“As SF is an acute phase protein, apparently normal levels may occur with iron deficiency in the context of an inflammatory disease process. A SF cut-off of 45 µg/L has been suggested as providing the optimal trade-off between sensitivity and specificity for iron deficiency in practice.”15
Ferinject efficacy
The efficacy of Ferinject was compared with that of oral ferrous sulphate in a prospective, randomised study in patients with anaemia secondary to non-variceal GI bleeding.9
Ferinject treatment resulted in:9
- rapid normalisation of mean Hb levels at 3 weeks after hospital discharge (85.7% of patients vs 45.2% of patients treated with ferrous sulphate; n=24/28 patients vs n=14/31 patients; p=0.001)
- complete response at the end of the study in 100% of patients (vs 61.3% of patient treated with ferrous sulphate; n=29/29 vs n=19/31; p=0.001)
- numerical decrease in the percentage of patients with mobility problems and pain/discomfort compared with an increase in the ferrous sulphate group (percentage of patients reporting mobility problems at Day 42: 8% in the Ferinject group (n=1/14) vs 20% in the ferrous sulphate group (n=4/19); percentage of patients reporting pain/discomfort at Day 42: 15% in the Ferinject group (n=2/14) vs 27% in the ferrous sulphate group (n=5/19); p=0.02)
Partial response rates over the duration of the study9*

Adapted from Ferrer-Barcelo L et al, 20199
*Primary outcome was complete response after 6 weeks; complete response = Hb levels ≥12 g/dL or ≥13 g/dL in women and men, respectively, at Day 42.
Results of quality of life questionnaire (EQ‐5D‐3L)

Adapted from Ferrer-Barcelo L et al, 20199

Adapted from Ferrer-Barcelo L et al, 20199
The EuroQoL 5 Dimensions (EQ‐5D‐3L) questionnaire was conducted face to face or via the telephone and assessed mobility, self-care, usual activities, anxiety/depression, and pain/discomfort. Higher percentages correspond to more patients with problems.9
The differences in pain/discomfort and mobility between the groups at day 42 were not significant.9
Ferinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions

Anaphylactic reactions are rare (≥1/10,000 to <1/1,000); fatalities have been reported. Hypersensitivity reactions are uncommon (≥1/1,000 to <1/100). There have been reports of hypersensitivity reactions which progressed to Kounis syndrome

Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each administration
If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution

Hypophosphataemia is a common (≥1/100 to <1/10) adverse drug reaction. Symptomatic hypophosphataemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post-marketing setting. Patients should be asked to seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors for hypophosphataemia. In case of persisting hypophosphataemia, treatment with ferric carboxymaltose should be re-evaluated
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Footnotes
ADR, adverse drug reaction; BSG, British Society of Gastroenterology; CKD, chronic kidney disease; ECCO, European Crohn's and Colitis Organisation, EQ-5D-3L, EuroQoL 5 Dimensions; GI, gastrointestinal; Hb, haemoglobin; HPP, hypophosphataemia; IBD, inflammatory bowel disease; ID, iron deficiency; IDA, iron deficiency anaemia, IV, intravenous; MCH, mean corpuscular haemoglobin; MCV, mean corpuscular volume; ns, non-significant; PRODIGGEST, hospital PROtocols to improve interDIsciplinary manaGEment of gaSTrointestinal diseases in hospital settings; RDW, red cell distribution width; RBC, red blood cell; SD, standard deviation; SF, serum ferritin; TSAT, transferrin saturation; WHO, World Health Organisation.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk
- PRODIGGEST (2017). Healthcare PROtocols to improve interDIsciplinary manaGEment of gaSTrointestinal diseases in hospital settings. Available at: https://www.aegastro.es/documents/prodiggest/Prodiggest-Management-of-anaemia-andiron-deficiency-in-gastrointestinal-bleeding.pdf. Accessed September 2025.
- Quinn EM, et al. Int J Surg 2017;38:1–8.
- Kaitha S, et al. World J Gastrointest Pathophysiol 2015;6(3):62–72.
- Gomollón F, Gisbert JP. World J Gastroenterol 2009;15(37):4659–4665.
- Khanbhal M, et al. ISRN Hematol 2014;2014:547914.
- Beattie WS, et al. Anesthesiology 2009;110(3):574–581.
- Tibi P, et al. Ann Thorac Surg 2021;112(3):981–1004.
- Ferrer-Barcelo L, et al. Aliment Pharamcol Ther 2019;50(3):258–268.
- Manning-Dimmitt LL, et al. Am Fam Physician 2005;71(7):1339–1346.
- World Health Organisation (WHO). Worldwide prevalence of anaemia 1993–2005. WHO Global Database on Anaemia. Available at: apps.who.int. Accessed September 2025.
- Bayraktar UD, Bayraktar S. World J Gastroenterol 2010;16(22):2720–2725.
- Villanueva C, et al. N Engl J Med 2013;368(1):11–21.
- Chen YC, et al. Clin Transl Gastroenterol 2018;9(3):138.
- Snook J, et al. Gut 2021;70:2030–2051.
- Diagnass AU, et al. J Crohns Colitis 2015:211–222.
FAQs
FERINJECT: FREQENTLY ASKED QUESTIONS

Can Ferinject be used in paediatric patients?
As of February 2023, Ferinject is approved for children and adolescent patients aged 1–13 years, as well as adult and adolescent patients aged 14 years and older.1
The efficacy and safety of Ferinject has not been investigated in children below 1 year of age. Ferinject is therefore not recommended for use in children in this age group.1
Learn moreHow may Ferinject benefit eligible patients with iron deficiency and chronic kidney disease?
Achieving higher ferritin levels with Ferinject in patients with iron deficiency and non‑dialysis chronic kidney disease (ND‑CKD) may lead to more effective iron deficiency anaemia (IDA) management versus achieving low ferritin levels with oral ferrous sulphate or intravenous Ferinject.2
In a 56‑week, open‑label, randomised, prospective, three‑arm study of patients with IDA and ND‑CKD (N=626), treating with Ferinject to achieve high ferritin levels (400–600 mcg/L) significantly reduced and/or delayed:2
- the need for alternative anaemia management
- the occurrence of a haemoglobin (Hb) trigger
Compared with achieving ferritin levels of 100–200 mcg/L using oral ferrous sulphate.2
The study primary endpoint was time to initiation of other anaemia management (ESA, other iron therapy or blood transfusion) or occurrence of an Hb trigger of two consecutive values <10 g/dL during Weeks 8–52.2
Learn more about Ferinject in patients with IDA and ND-CKDHow may Ferinject benefit eligible patients with iron deficiency in heart failure?
Iron deficiency in heart failure1
- In a multi-centre, double‑blind, randomised, placebo‑controlled trial in patients stabilised for acute heart failure (HF; N=1,108) with concomitant iron deficiency and a left ventricular ejection fraction <50%, Ferinject reduced the rate of the composite primary endpoint of cardiovascular death and HF re-hospitalisations versus placebo, but this was not statistically significant. The trial did not meet its primary endpoint of recurrent hospitalisation for HF or cardiovascular death.3
- Results from a meta-analysis suggested that administration of intravenous iron versus placebo/standard of care to treat iron deficiency in patients with HF may reduce the risk of HF hospitalisation.4
What are the dosing requirements for Ferinject in children aged 1–13 years?
Ferinject can be delivered by infusion or injection in 15 minutes, which must be followed by a 30-minute observation period:1
- A maximum of 15 mg/kg up to 750 mg in a single administration
- Ferinject is not recommended for use in children aged 1–13 years with chronic kidney disease (CKD) requiring haemodialysis
Do not administer more than 750 mg (15 mL) of Ferinject per week. If the total iron need is higher, then the administration of an additional dose should be a minimum of 7 days apart from the first dose.1
The efficacy and safety of Ferinject has not been investigated in children below 1 year of age. Ferinject is therefore not recommended for use in children in this age group.1
Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available and where full resuscitation facilities can be assured. The patient should be observed for adverse effects during and for at least 30 minutes following each Ferinject administration, especially for signs and symptoms of hypersensitivity reactions.1
Download a paediatric dosing cardWhat are the dosing requirements for Ferinject in patients aged 14+ years?
Ferinject delivers up to 1,000 mg of intravenous iron to your patients aged 14 years and older1
Determination of the total iron need1
Ferinject cumulative dosing based on Hb value and body weight; two doses may be required to replenish the total iron need:
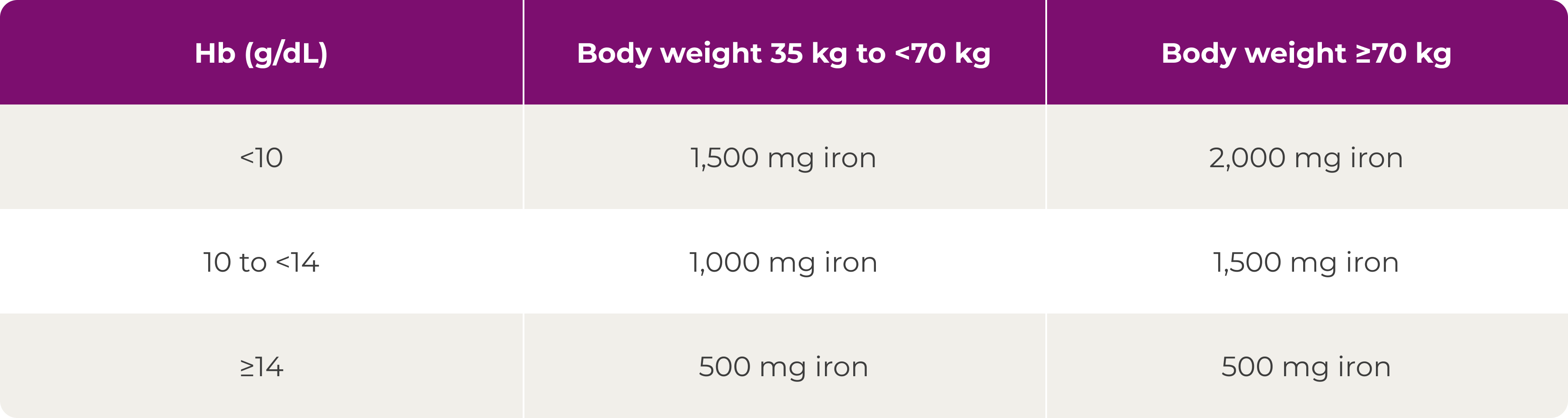
Ferinject can be delivered by infusion or injection in 15 minutes, which must be followed by a 30‑minute observation period:1
- A maximum of 20 mg/kg up to 1,000 mg in a single infusion
- A maximum of 15 mg/kg up to 1,000 mg in a single injection
- A single maximum daily dose of 200 mg iron should not be exceeded in patients with haemodialysis‑dependent CKD
Do not administer more than 1000 mg (20 mL) of Ferinject per week. If the total iron need is higher, then the administration of an additional dose should be a minimum of 7 days apart from the first dose.1
Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available and where full resuscitation facilities can be assured. The patient should be observed for adverse effects during and for at least 30 minutes following each Ferinject administration, especially for signs and symptoms of hypersensitivity reactions.1
Download a dosing cardIs hypophosphataemia a potential adverse drug reaction with Ferinject?
Yes, hypophosphataemia (HPP) is a common (≥1/100 to <1/10) adverse drug reaction after treatment with Ferinject1
Risk of HPP5,6
Certain groups of patients are at a higher risk of developing symptomatic HPP after Ferinject administration than others.
Risk factors for developing symptomatic HPP after Ferinject administration include:5,6
- Vitamin D deficiency
- Severe iron deficiency*
- Calcium malabsorption
- Multiple Ferinject doses in patients requiring repeated high dosing
- Secondary hyperparathyroidism in high-risk population†
- Symptomatic HPP leading to osteomalacia and fractures has been reported in the post-marketing setting1
*Low ferritin and ongoing blood loss mainly in patients with gastrointestinal or gynaecological conditions.6
†High-risk populations include Black/African race and high body mass index.5
Serum phosphate should be monitored in patients who receive long‑term treatment or multiple administrations at higher doses, and in those with existing risk factors for HPP1
In case of persisting HPP, treatment with Ferinject should be re‑evaluated.1
What guidance is provided around potential hypersensitivity reactions?
Hypersensitivity reactions with Ferinject1
Parenterally administered iron preparations can cause hypersensitivity reactions including serious and potentially fatal anaphylactic reactions. Hypersensitivity reactions have also been reported after previously uneventful doses of parenteral iron complexes. There have been reports of hypersensitivity reactions which progressed to Kounis syndrome (acute allergic coronary arteriospasm that can result in myocardial infarction).
The risk is enhanced for patients with known allergies including drug allergies, including patients with a history of severe asthma, eczema or other atopic allergy.
There is also an increased risk of hypersensitivity reactions to parenteral iron complexes in patients with immune or inflammatory conditions (e.g. systemic lupus erythematosus, rheumatoid arthritis).
Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions are immediately available, in an environment where full resuscitation facilities can be assured. Each patient should be observed for adverse effects for at least 30 minutes following each Ferinject administration. If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardiorespiratory resuscitation and equipment for handling acute anaphylactic reactions should be available, including an injectable 1:1,000 adrenaline solution. Additional treatment with antihistamines and/or corticosteroids should be given as appropriate.
The use of Ferinject is contraindicated in cases of hypersensitivity to the active substance, to Ferinject or any of its excipients, or known serious hypersensitivity to other parenteral iron products1
How should Ferinject be stored?
Ferinject storage1
Store in the original package to protect it from light. Do not store above 30°C.
Do not freeze.
Shelf life after first opening of the container:
- From a microbiological point of view, preparations for parenteral administration should be used immediately
- If not used immediately, in-use storage times and conditions are the responsibility of the user. Administration of the product must be carried out under controlled and validated aseptic conditions
- Chemical and physical in-use stability has been demonstrated for 7 days at 30°C
Shelf life in polyethylene and polypropylene containers after dilution with sterile 0.9% m/V sodium chloride solution:
- From a microbiological point of view, preparations for parenteral administration should be used immediately after dilution with sterile 0.9% m/V sodium chloride solution
- If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2–8°C
- Chemical and physical in-use stability has been demonstrated for 72 hours at 30°C at concentrations of 2 mg/mL and 5 mg/mL
Shelf life in polypropylene syringe (undiluted):
- From a microbiological point of view, the product should be used immediately
- If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2–8°C
- Chemical and physical in-use stability has been demonstrated for 72 hours at 30°C
Can Ferinject stain the skin?
Yes, skin discolouration is a rare (≥1/10,000 to <1/1,000) adverse drug reaction after treatment with Ferinject1
Caution should be exercised to avoid paravenous leakage when administering Ferinject. Paravenous leakage of Ferinject at the administration site may lead to irritation of the skin and potentially long‑lasting brown discolouration at the site of administration. In case of paravenous leakage, the administration of Ferinject must be stopped immediately.1
Flushing the cannula site with saline before and after intravenous iron infusion can be used as a precautionary measure for skin discolouration.7
Are any patient information leaflets available for Ferinject?
Yes, patient information leaflets are available. Download a patient leaflet and a parent/caregiver leaflet below.
Patient information leafletFerinject has a well-characterised tolerability profile:1
Most common adverse drug reactions (ADRs): nausea, injection/infusion site reactions, hypophosphataemia, headache, flushing, dizziness and hypertension. Most serious ADR: anaphylactic reactions
Please refer to the Ferinject Summary of Product Characteristics for complete tolerability information.
References & footnotes
Abbreviations
ADR, adverse drug reaction; CKD; chronic kidney disease; ESA, erythropeiesis-stimulating agent; Hb, haemoglobin; HF, heart failure; HPP, hypophosphataemia; IDA, iron deficiency anaemia; ND-CKD, non-dialysis chronic kidney disease.
References
- Ferinject Summary of Product Characteristics. Available at: www.medicines.org.uk
- Macdougall I C, et al. Nephrol Dial Transplant 2014;29:2075–84 (+ suppl).
- Ponikowski P, et al. Lancet 2020;396:1895–904.
- Graham F J, et al. Eur J Heart Fail 2023;25:528–37.
- Rosano G, et al. J Clin Med 2020;9(11):3587.
- Schaefer B, et al. Bone 2022;154:116202.
- Crowley C M, et al. BMJ Case Rep 2019;12:e229113.
UK-FCM-2500185 | Date of preparation: October 2025
UK-FCM-2100120 (v4.0) | Date of preparation: March 2025
UK-FCM-2300250 (v2.0) | Date of preparation: March 2025
UK-FCM-2200178 (v2.0) | Date of preparation: March 2025
UK-FCM-2300249 (v3.0) | Date of preparation: September 2025
UK-FCM-2500147 | Date of preparation: September 2025
UK-FCM-2500146 | Date of preparation: August 2025
UK-FCM-2500145 | Date of preparation: August 2025
UK-FCM-2500144 | Date of preparation: September 2025
UK-FCM-2500143 | Date of preparation: August 2025
UK-FCM-2500160 | Date of preparation: September 2025
UK-FCM-2500142 | Date of preparation: August 2025
UK-FCM-2100140 (v7.0) | Date of preparation: July 2025
Adverse events should be reported. Reporting forms and information for the United Kingdom can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Vifor Fresenius Medical Care Renal Pharma, care of Vifor Pharma Ltd.
Tel: +44 1276 853633. E-mail: MedicalInfo_UK@viforpharma.com.

Stay informed
Register with CSL for the latest releases. This will include promotional content
Register