
FILSPARI®▼
(sparsentan)
FOUNDATION REINVENTED WITH DUAL ACTION in primary IgA nephropathy1–3
Prescribing information (200 mg) Prescribing information (400 mg)
FILSPARI®▼
(sparsentan)
FOUNDATION REINVENTED WITH DUAL ACTION in primary IgA nephropathy1–3
Prescribing information (200 mg) Prescribing information (400 mg)FILSPARI® (sparsentan) is indicated for the treatment of adults with primary immunoglobulin A nephropathy (IgAN) with a urine protein excretion ≥1.0 g/day (or urine protein-to-creatinine ratio ≥0.75 g/g)1
About IgAN
IgA nephropathy (IgAN) may cause irreversible kidney damage in your patients4–6
IgAN is a major cause of kidney failure7–9
IgAN is the most common primary glomerulonephritis worldwide.7,8 In the UK, >1 in 10 patients (13.7%) having in-centre haemodialsysis have glomerulonephritis10
A systemic disease that results in damage to the kidney, IgAN is a leading cause of CKD and end-stage kidney disease (ESKD) globally7–9

IgAN has a global annual incidence of
2.5 per 100,000 population11
It is most common in people of East Asian ancestry followed by Caucasians8,11

Most patients are diagnosed in their
30s to 40s12–14

Around two-thirds of patients have CKD
stage ≥3 at diagnosis4,15
Patients with IgAN (adult diagnosis) reach kidney failure at a mean of 49 years4
Retrospective cohort study: real-world evidence from the IgAN cohort of the UK National RaDaR* initiative confirmed that patients with IgAN have a high risk of kidney failure4
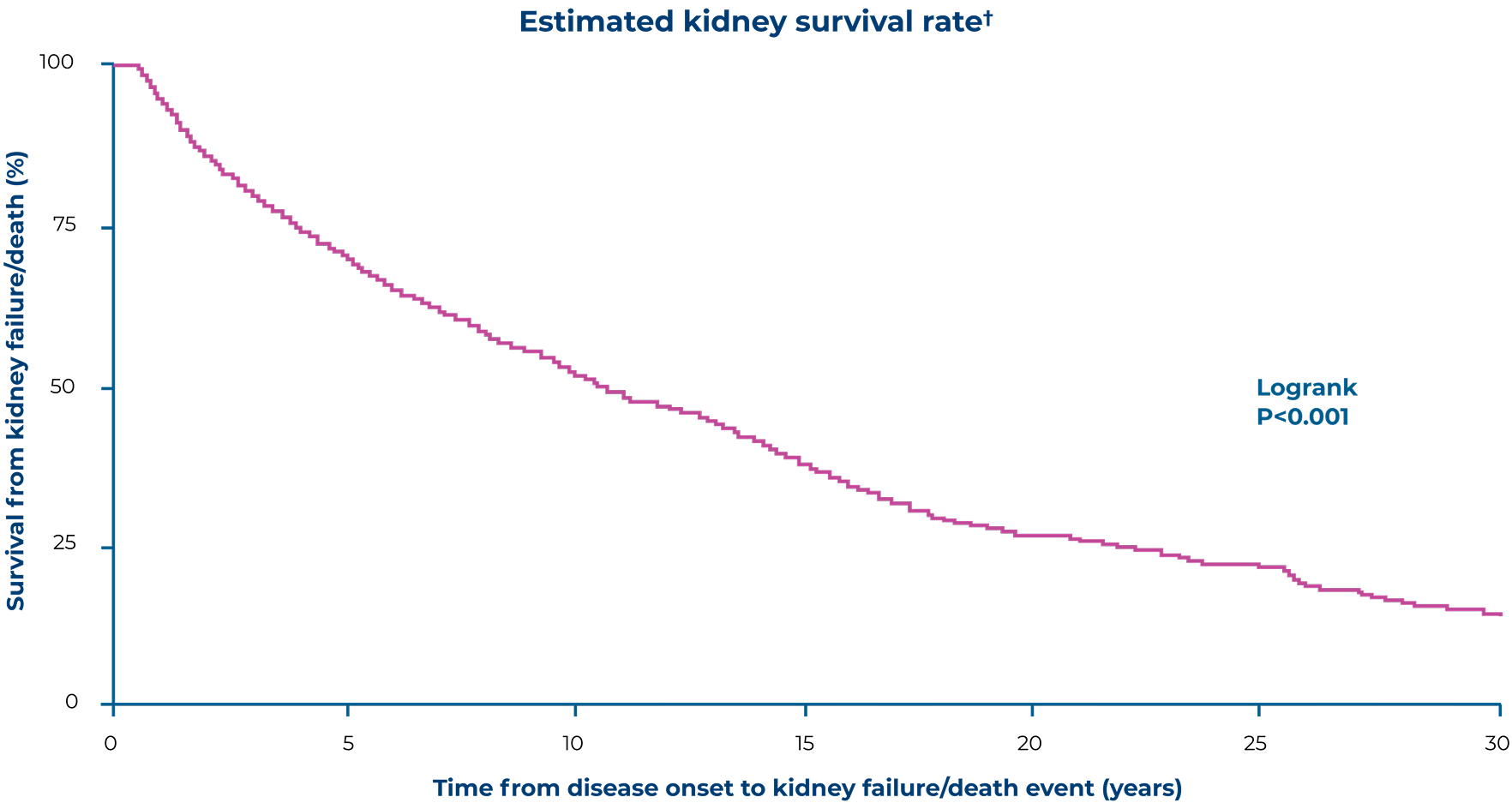
Adapted from Pitcher D, et al. 20234
Patients enrolled had a biopsy-proven diagnosis of IgAN plus proteinuria >0.5 g/day or eGFR <60mL/min/1.73 m2 at any time in the history of their disease4
In the RaDaR cohort of adult patients with IgAN (n=2,299):4

Mean age at diagnosis

Median follow-up time
Of patients reached kidney failure or died during
the study period

After diagnosis, most patients progressed to kidney failure within this timeframe
*RaDaR is a UK-based rare kidney disease registry, the IgAN cohort (N=2,439; 2,999 adults and 140 children) had biopsy-proven IgAN with proteinuria >0.5 g/day or eGFR <60 mL/min/1.73 m2 at any time in the history of their disease. Patients had CKD at baseline4
†Kidney survival was defined as the absence of either kidney failure or death4
The KDIGO 2025 guidelines for IgAN updates the management and treatment recommendations16
The KDIGO 2025 guidelines for IgAN emphasise the need to reduce proteinuria in patients16

Patients are considered at risk of progressive loss of kidney function if they have proteinuria ≥0.5 g/day while on or off treatment for IgAN16
Updated kidney biopsy threshold:16
IgAN can only be diagnosed with a kidney biopsy; to ensure early diagnosis and prompt treatment, a kidney biopsy should be considered in all adults with proteinuria ≥0.5 g/day in whom IgAN is a possible diagnosis and kidney biopsy is not contraindicated
Treatment goals in patients with IgAN at risk of progressive loss of kidney function:16
- Reduce the rate of loss of kidney function to <1 mL/min/year for the rest of the patient’s life
- Urine protein excretion should be maintained at <0.5 g/day, and ideally <0.3 g/day*

Urine protein excretion should be maintained at <0.5 g/day, and ideally <0.3 g/day*
Reduce the rate of loss of kidney function to <1 mL/min/year for the rest of the patient’s life
The updated guidelines also recommend that the focus of management in most patients should be to simultaneously:16
Prevent or reduce IgA immune complex (IgA-IC) formation and immune complex-mediated glomerular injury
Manage the consequences of existing IgAN-induced nephron loss
KDIGO 2025 treatment targets in IgAN and available to-date approved treatment options16
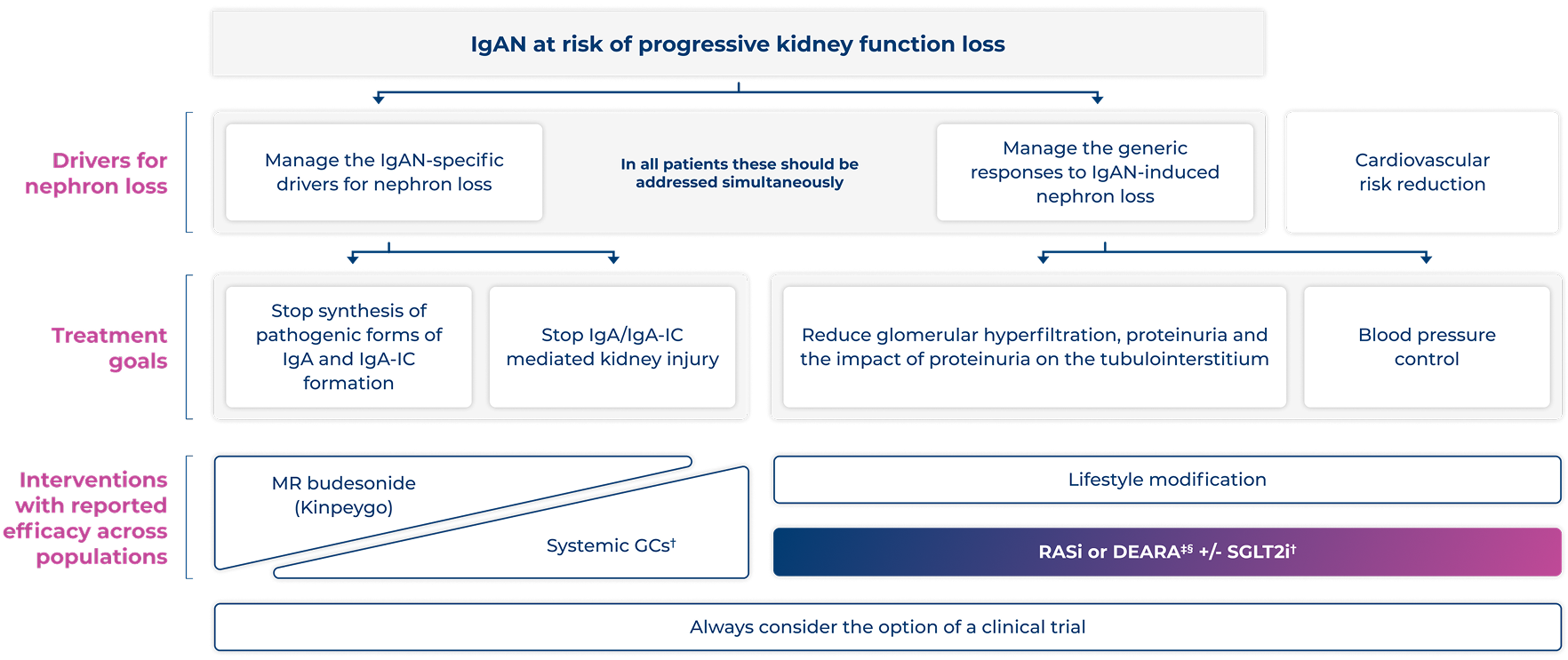
Adapted from KDIGO. 202516
*In some patients with extensive kidney scarring this may not be possible and multiple drugs are likely to be needed to achieve this16
†Systemic GCs and SGLT2i are not licensed in the UK for the treatment of IgAN
‡FILSPARI is indicated for the treatment of adults with primary IgAN with a UPE ≥1.0 g/day (Or UP/C ≥0.75 g/g)1
§Practice point 1.4.4.3: FILSPARI is a DEARA and should not be prescribed together with RASi16
Two critical pathways are involved in disease progression7,17–19
Two critical pathways are involved in disease
progression7,17–19
The mutual upregulation of ET-1 and ANG II is involved in the pathogenesis of IgAN7,17–19

To prevent further nephron loss in IgAN, treatment should address both the CKD and immunological dimensions of IgAN3
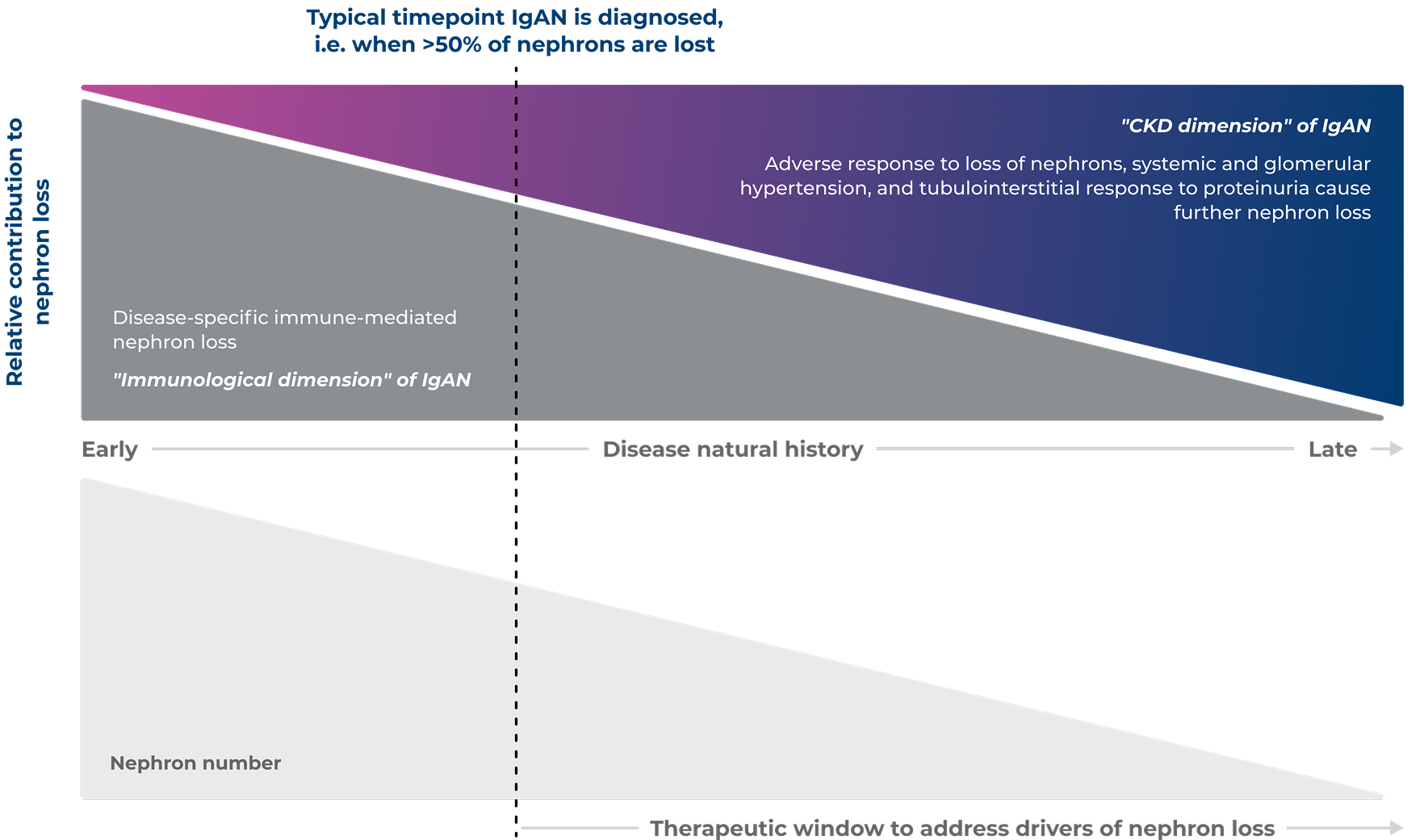
Adapted from Barratt J, et al. 20243
As a DEARA, targeting both endothelin and angiotensin pathways involved in IgAN disease progression, FILSPARI is a foundational treatment in optimised kidney care for IgAN1,3,19
Click here to learn more about the efficacy of FILSPARI
References & footnotes
Abbreviations
ACEi, angiotensin-converting enzyme inhibitor; ANG II, angiotensin II; ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; DEARA, dual endothelin angiotensin receptor antagonist; ECM, extracellular matrix; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; ET-1, endothelin 1; GC, glucocorticoid; IgA, immunoglobulin A; IgA-IC, immunoglobulin A immune complex; IgAN, immunoglobulin A nephropathy; KDIGO, Kidney Disease: Improving Global Outcomes; MR, modified release; Na, sodium; RaDaR, UK National Registry of Rare Kidney Diseases; RASi, renin-angiotensin system inhibitor; SGLT2i, sodium-glucose co‑transporter-2 inhibitor; UK, United Kingdom.
References
- FILSPARI SmPC.
- Rovin B, et al. Lancet. 2023;402(10417):2077–90.
- Barratt J, et al. Front Med. 2024;11:1461879.
- Pitcher D, et al. Clin J Am Soc Nephrol. 2023;18(6):727–38.
- Reich HN, et al. J Am Soc Nephrol.2007;18:3177–83.
- ElHafeez SA, et al. Nephrol Dial Transplant. 2024;39(9):1449–60.
- Lai KN, et al. Nat Rev Dis Primers. 2016;2:16001.
- Yeo S, et al. Pediatr Nephrol. 2018;33:763–77.
- Wyatt R, Julian B. N Engl J Med. 2013;368:2402–14.
- UK Kidney Association. UK Renal Registry. 26th Annual Report. Data to 31/12/2022. Available at: https://www.ukkidney.org/audit-research/annual-report/26th-annual-report-data-31122022. Date accessed: October 2025.
- McGrogan A, et al. Nephrol Dial Transplant. 2011;26:414–30.
- Nair R, et al. Kidney Int. 2006;69:1455–8.
- Barratt J, Feehally J. J Am Soc Nephrol. 2005;16(7):2088–97.
- O’Shaughnessy MM, et al. Clin J Am Soc Nephrol. 2017;12(4):614–23.
- Caster D, et al. Kidney Int Reports. 2023;8:1792–800.
- Kidney Disease: Improving Global Outcomes (KDIGO) IgAN and IgAV Work Group. Kidney Int. 2025;108(4S):S1−S71.
- Lehrke I, et al. J Am Soc Nephrol. 2001;12(11):2321–9.
- Chan LY, et al. J Am Soc Nephrol. 2005;16(8):2306–17.
- Komers R, Plotkin H. Am J Physiol Regul Integr Comp Physiol. 2016;310:R877‒84.
- Kohan DE, et al. Compr Physiol. 2011;1:883–919.
- Raina R, et al. Kidney Dis. 2020;6:22–34.
- Sharma S, Smyth B. Kidney Blood Press Res. 2021;46:411–20.

FILSPARI is contraindicated during pregnancy. FILSPARI treatment must only be initiated in women of childbearing potential when the absence of pregnancy has been verified. Women of childbearing potential have to use effective contraception during and up to 1 month after treatment has stopped.
▼Adverse events should be reported. Reporting forms and information for the United Kingdom can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Vifor Pharma Ltd.
Tel: +44 1276 853633. E‑mail: MedicalInfo_UK@viforpharma.com
Please read the full SmPC prior to administration. Filspari® is a registered trademark.
UK-SPT-2500168 | Date of preparation: October 2025
Efficacy
FILSPARI is the only UK-licensed IgAN treatment evaluated head-to-head against irbesartan2,4,5
Significant and superior proteinuria reduction vs irbesartan2,4
Primary endpointFILSPARI delivered significant and superior proteinuria reduction at Week 36 vs irbesartan2,4
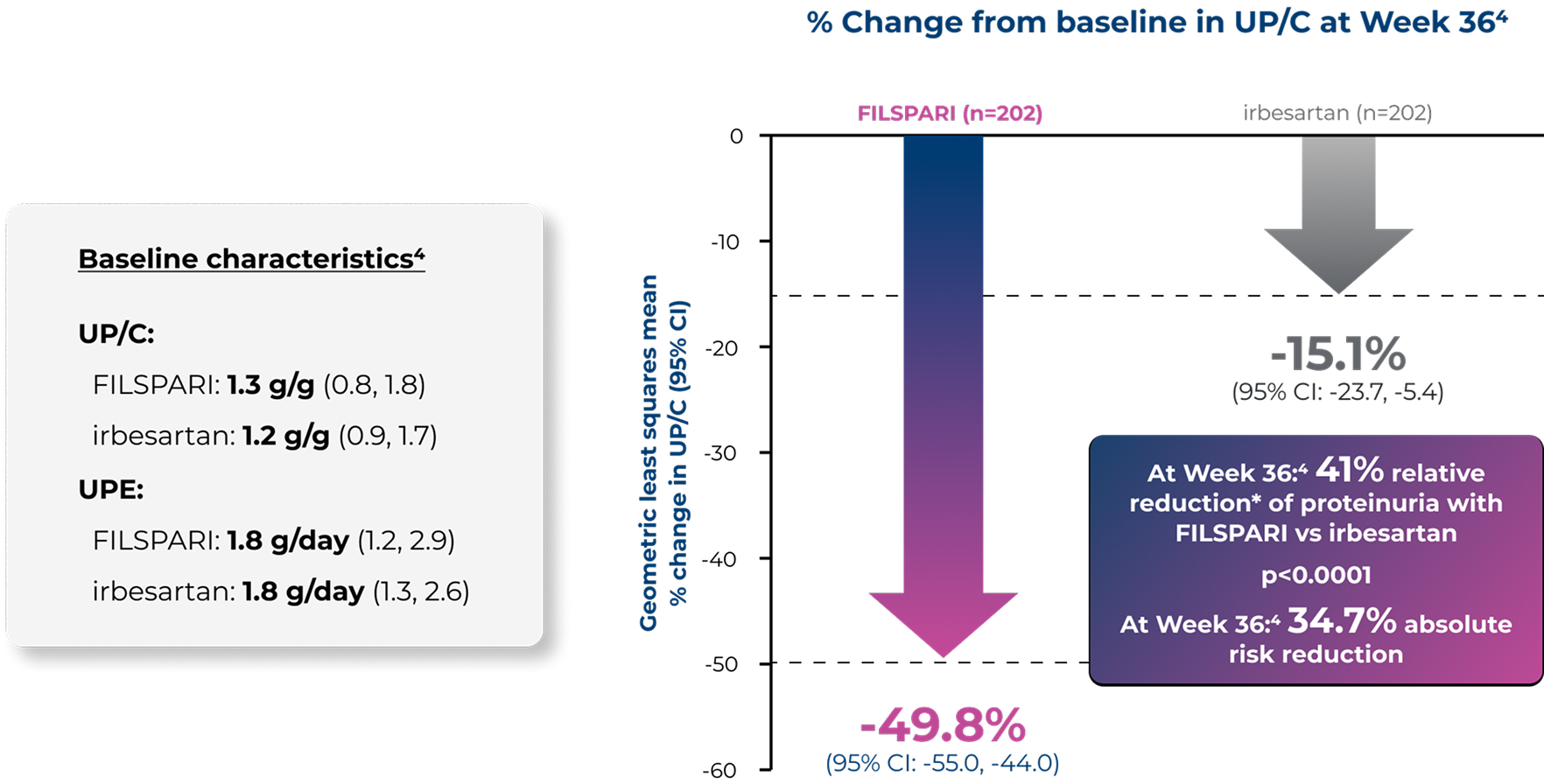
Adapted from Heerspink HJL, et al. 20234
*Geometric LS mean ratio; 95% CI, 0.51, 0.694
FILSPARI delivered significant and superior proteinuria reduction at Week 36 vs irbesartan2,4
Adapted from Heerspink HJL, et al. 20234
*Geometric LS mean ratio; 95% CI, 0.51, 0.694
Significantly slower rates of eGFR decline vs irbesartan2*
Secondary endpointFILSPARI significantly slowed the rate of eGFR decline vs irbesartan from Week 6 to Week 1102*
eGFR by visit to Week 1142†
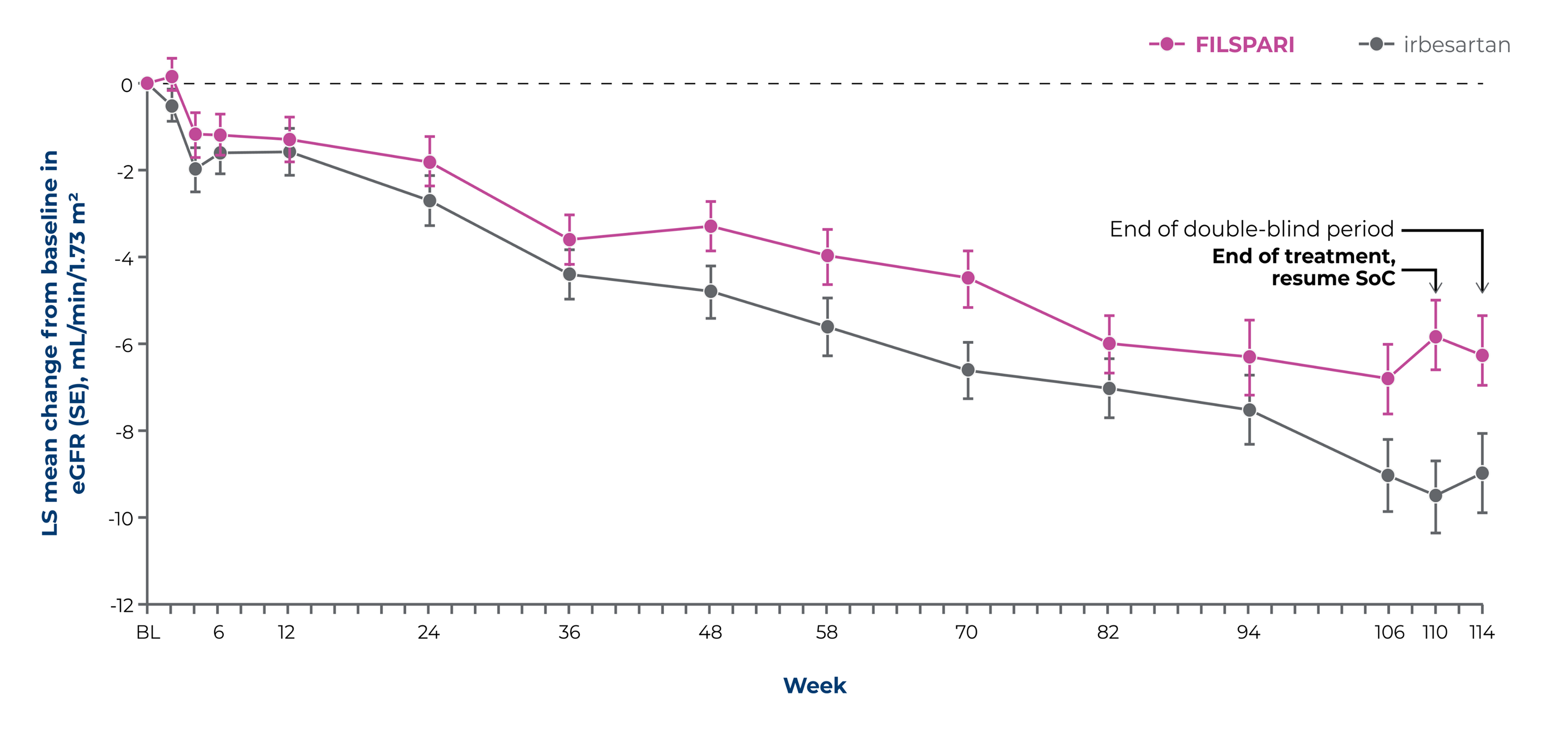
Baseline eGFR, mL/min/1.73 m2, mean (SD): FILSPARI: 56.8 (24.3) irbesartan: 57.1 (23.6)
Adapted from Rovin, et al. 20232

*Chronic slope2
†Key secondary endpoints included chronic eGFR slope (rate of eGFR change from Week 6 to Week 110) and total eGFR slope (Day 1–Week 110) over the full double-blind treatment period2
Extrapolation of eGFR slopes for FILSPARI and irbesartan using baseline eGFR2,6
Extrapolated dataOutcomes presented are not efficacy outcomes and should be validated in the future with long-term data as it becomes available
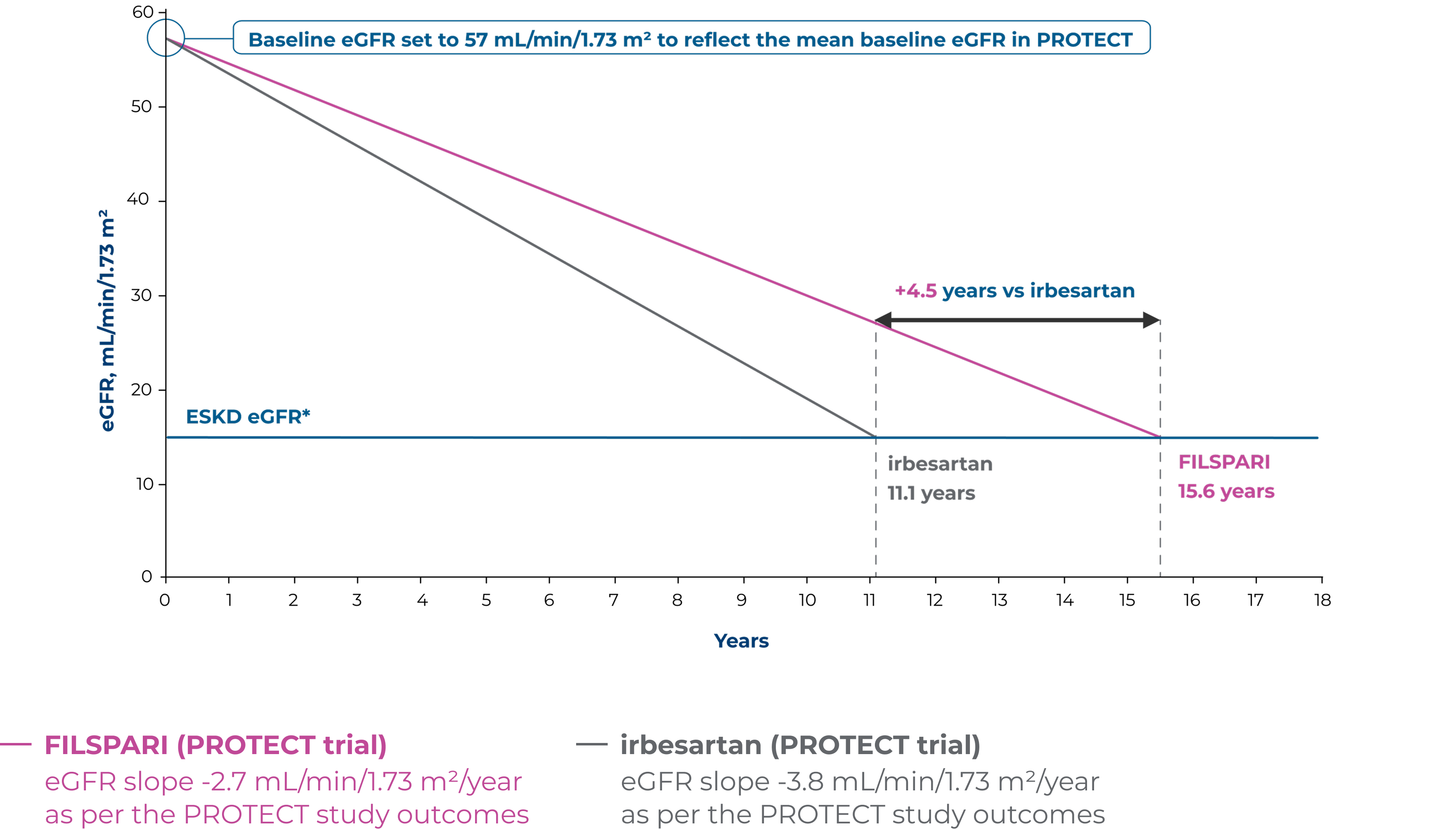
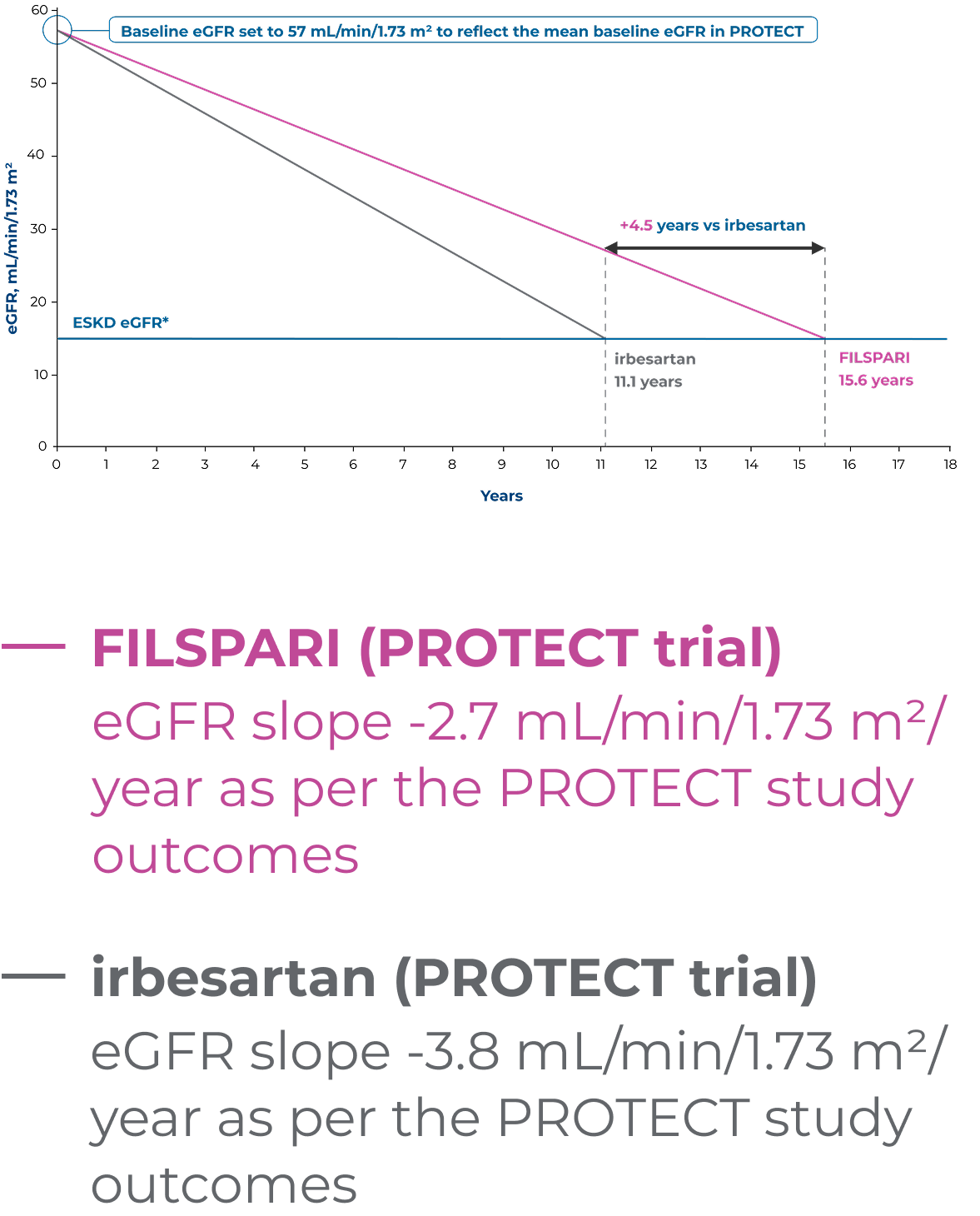
Adapted from Rovin B, et al. 2023 [Suppl Appendix]6
Based on linear extrapolation of the eGFR slopes calculated in the PROTECT study, the theoretical effect of treatment on the time to kidney failure for a patient with IgAN can be illustrated2,6
These data are extrapolated and therefore act under the assumption that eGFR decline is constant and decreases in a linear fashion in the course of the disease, which is not necessarily accurate for real-world, long-term effects. Outcomes may vary on an individual basis.
*End-stage kidney disease (ESKD) eGFR defined as Stage 5 kidney disease (<15 mL/min/1.73 m2)6
Absolute difference in change in eGFR from baseline to Week 110 was numerically better for FILSPARI vs irbesartan2
Other secondary endpointAbsolute difference in change in eGFR from baseline to Week 110:2
3.7 mL/min/1.73 m2
(95% CI; 1.5, 6.0)
LS mean change in eGFR from baseline to Week 110:2

FILSPARI
-5.8
mL/min/1.73 m2
(95% CI; -7.4, -4.2)
VS

irbesartan
-9.5
mL/min/1.73 m2
(95% CI; -11.2, -7.9)
Statistical hypotheses for other prespecified secondary efficacy endpoints were not formally tested under the hierarchical testing procedure because the p value for the eGFR total slope was more than 0.052
Under the hierarchical testing procedure, this study did not formally test the statistical hypotheses for other prespecified secondary efficacy endpoints, as significance was narrowly missed for the eGFR total slope. For these and other efficacy endpoints, nominal 95% CIs are presented2
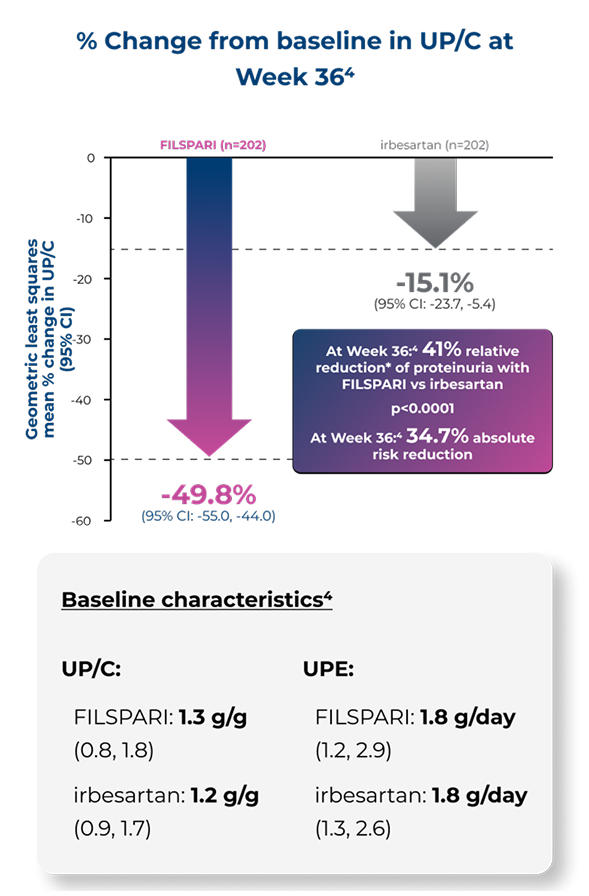
Proteinuria reduction at Week 110 vs irbesartan2,4
Other secondary endpointA numerical difference in proteinuria reduction for FILSPARI was observed through Week 110 vs irbesartan2,4
The reduction of proteinuria at Week 36 was sustained through Week 110 vs irbesartan2
% Change from baseline in UP/C to Week 1102
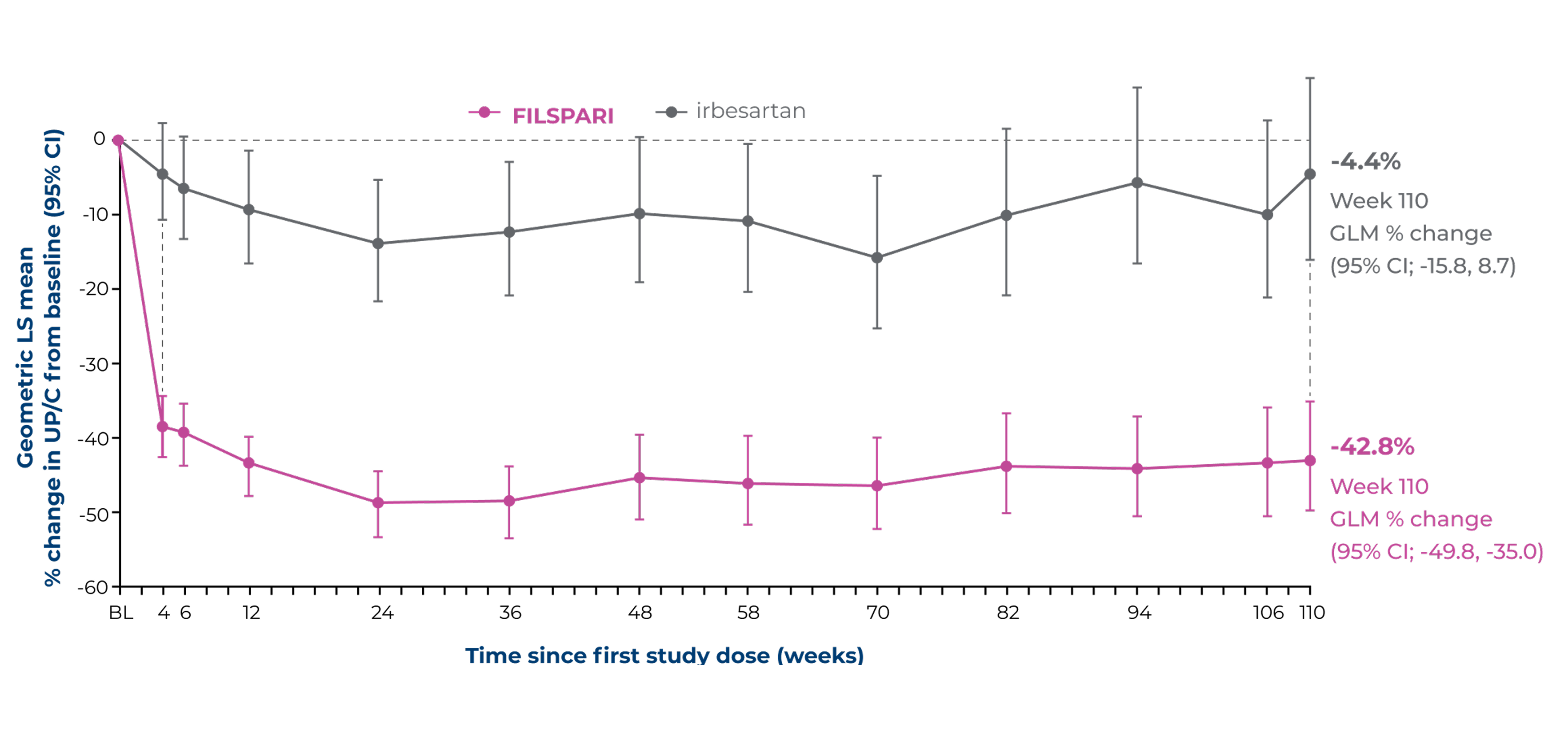
Adapted from Rovin B, et al. 2023 [Suppl Appendix]6
Under the hierarchical testing procedure, the study did not formally test the statistical hypotheses for 'other' prespecified secondary efficacy endpoints. No definite conclusions can be drawn from these data2
Proportion of patients reaching the composite kidney failure endpoint* vs irbesartan2
Other secondary endpointTime to reach the composite kidney failure endpoint2*
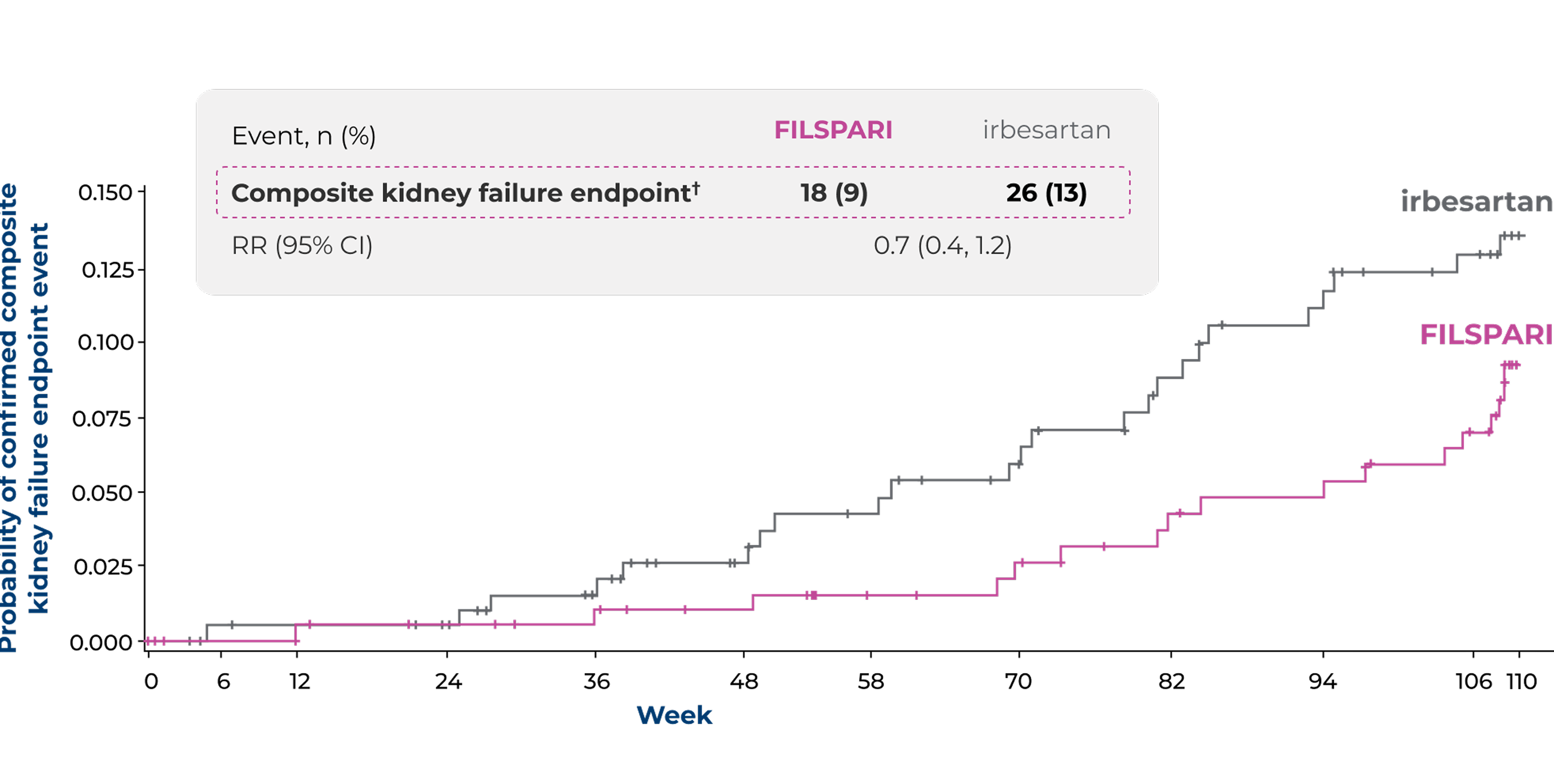
Vertical bars indicate censored patients
Adapted from Rovin B, et al. 20232
Under the hierarchical testing procedure, the study did not formally test the statistical hypotheses for 'other' prespecified secondary efficacy endpoints. No definite conclusions can be drawn from these data2
*The composite kidney failure endpoint was defined as confirmed 40% eGFR reduction, ESKD (defined as initiation of renal replacement therapy or sustained eGFR <15 mL/min/1.73 m2) or all-cause mortality2
†Patients with confirmed 40% reduction in eGFR (irbesartan, n=22 [11%]; FILSPARI, n=18 [9%]), ESKD (irbesartan, n=11 [5%]; FILSPARI, n=9 [4%]) or death (irbesartan, n=1 [<1%]; FILSPARI, n=0)2
Proteinuria remission with FILSPARI vs irbesartan2,6
Exploratory/post-hoc endpointProportion of patients achieving complete (<0.3 g/day) or partial (<1.0 g/day) proteinuria remission at any point up to Week 1102,6
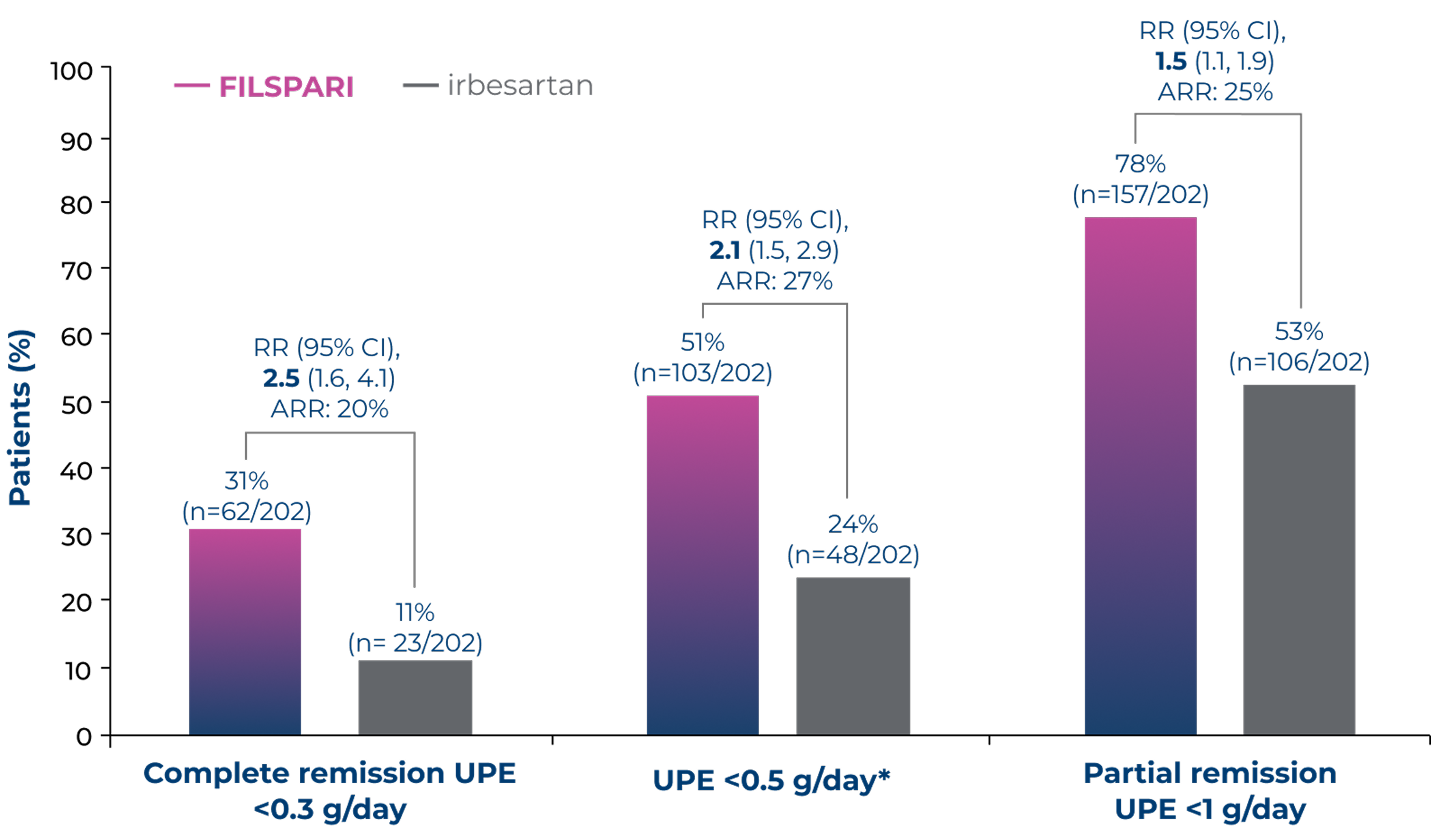
Adapted from Rovin B, et al. 2023 [Suppl Appendix]6
Under the hierarchical testing procedure, the study did not formally test the statistical hypotheses for exploratory/post-hoc efficacy endpoints. No definite conclusions can be drawn from these data2
*The proportion of patients achieving UPE <0.5 g/day was a post-hoc assessment6
Proportion of patients requiring systemic immunosuppressive medication with FILSPARI vs irbesartan2,6
Exploratory endpointLess frequent initiation of rescue immunosuppressive medications with FILSPARI vs irbesartan2
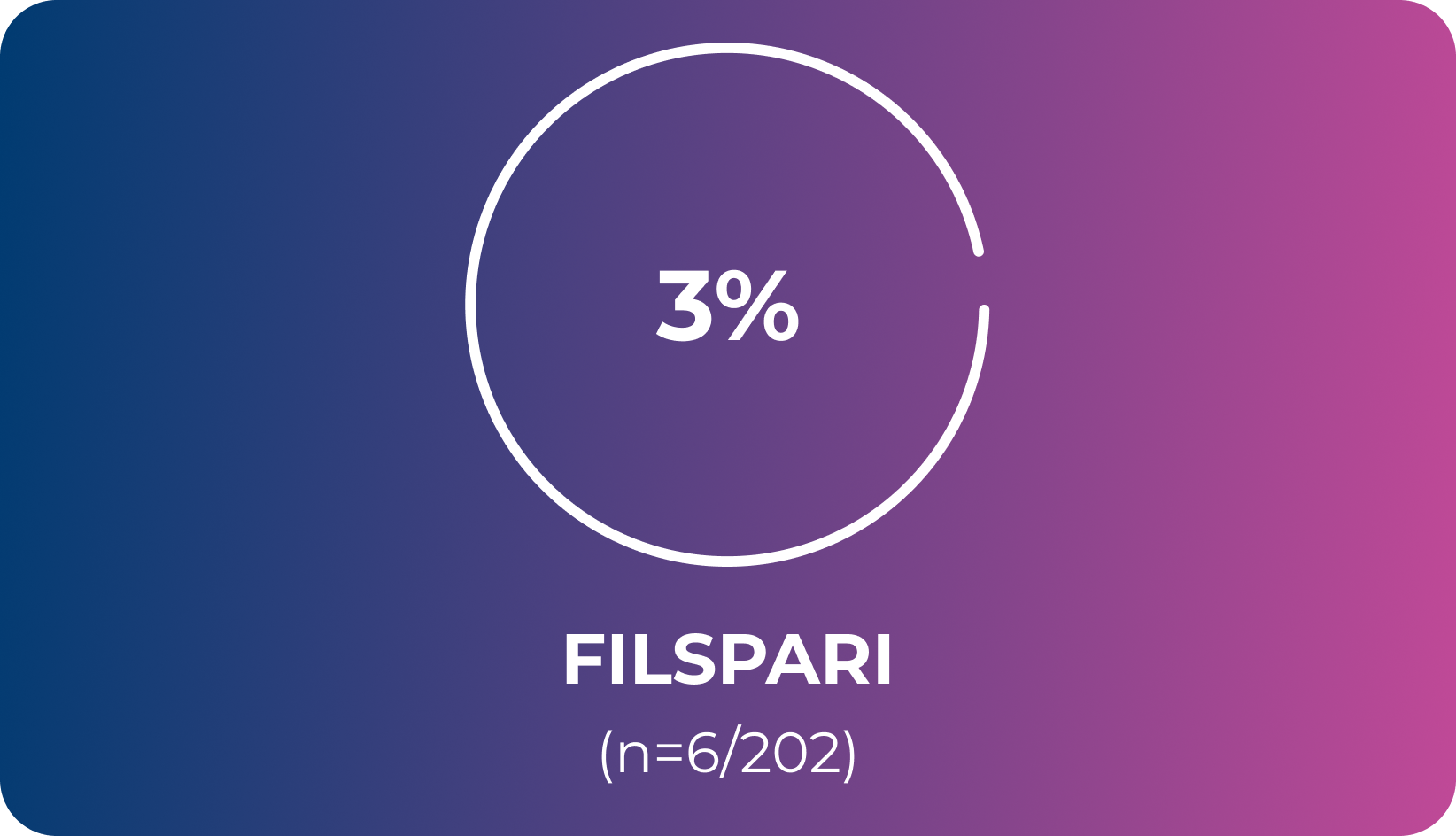

OR; 95% CI, 2.87 (1.09–7.57)6
Under the hierarchical testing procedure, the study did not formally test the statistical hypotheses for exploratory efficacy endpoints. No definite conclusions can be drawn from these data2
References & footnotes
Abbreviations
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARR, absolute relative risk; AT1, angiotensin II type 1; BL, baseline; BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; ETA, endothelin type A; GLM, geometric least-squares mean; IgA, immunoglobulin A; IgAN, immunoglobulin A nephropathy; IQR, interquartile range; LS, least-squares; MoA, mechanism of action; OLE, open-label extension; OR, odds ratio; RASi, renin-angiotensin system inhibitor; RR, relative risk; SD, standard deviation; SE, standard error; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SoC, standard of care; TEAE, treatment-emergent adverse event; UK, United Kingdom; UP/C, urine protein-to-creatinine ratio; UPE, urine protein excretion.
References
- FILSPARI SmPC.
- Rovin B, et al. Lancet. 2023;402(10417):2077–90.
- Barratt J, et al. Front Med. 2024;11:1461879.
- Heerspink HJL, et al. Lancet. 2023;13. 401(10388):1584–94.
- Campbell KN, et al. Int J Nephrol Renovasc Dis. 2023;16:281–91.
- Rovin B, et al. Lancet. 2023;402(10417):2077–90. [Suppl Appendix].
- PROTECT ClinicalTrials.gov: Available at: http://clinicaltrials.gov/ct2/show/NCT03762850.
Date accessed: November 2025.
Additional
DE-FCM-2100195

FILSPARI is contraindicated during pregnancy. FILSPARI treatment must only be initiated in women of childbearing potential when the absence of pregnancy has been verified. Women of childbearing potential have to use effective contraception during and up to 1 month after treatment has stopped.
▼Adverse events should be reported. Reporting forms and information for the United Kingdom can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Vifor Pharma Ltd.
Tel: +44 1276 853633. E‑mail: MedicalInfo_UK@viforpharma.com
Please read the full SmPC prior to administration. FILSPARI® is a registered trademark.
UK-SPT-2500167 (v2.0) | Date of preparation: November 2025
Safety, dosing and administration
Safety profile of FILSPARI1
The most common adverse drug reactions observed during clinical trials1
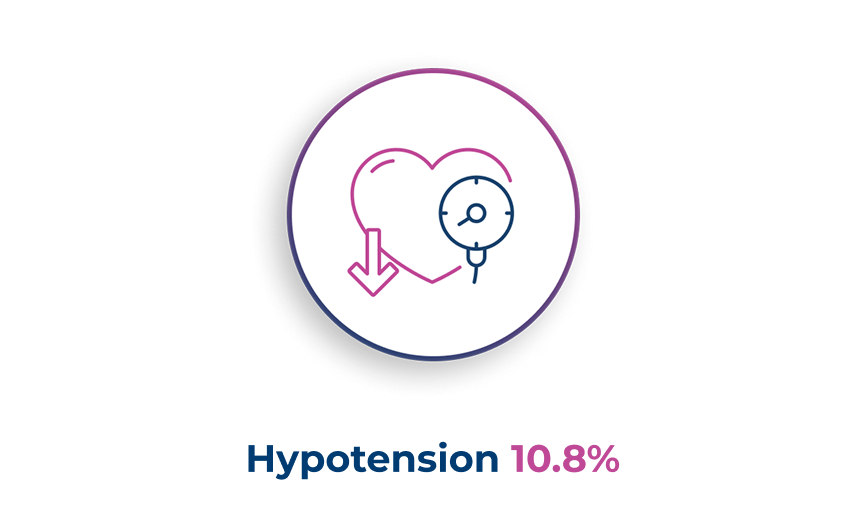



The most common serious adverse reaction reported was acute kidney injury (0.9%)1
Adverse drug reactions observed during clinical trials1*
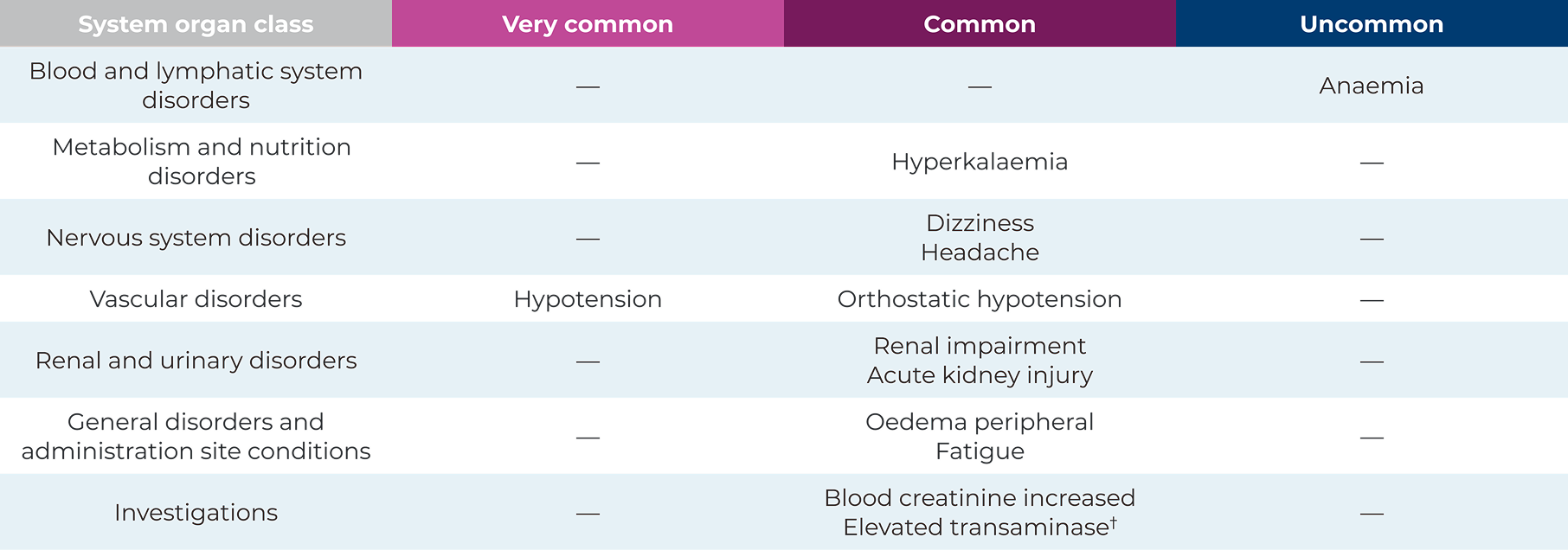
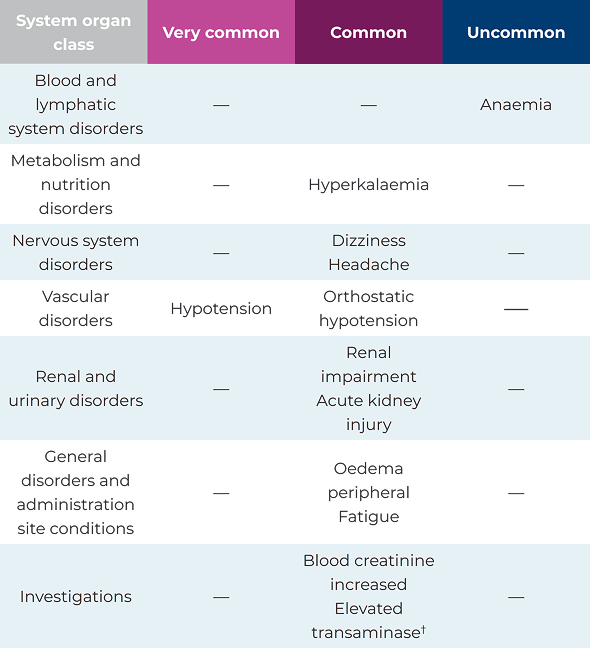
*Elevated transaminase includes preferred terms of alanine aminotransferase increased, aspartate aminotransferase increased, gamma‑glutamyltransferase increased and hepatic enzyme increased1
One tablet, once daily. One molecule addressing two critical pathways involved in disease progression1


200 mg or 400 mg tablets


1x daily


With or without food
It is recommended to swallow the tablets whole with water to avoid bitter taste


Only initiate FILSPARI in verified absence of pregnancy and while on effective contraception
Posology1


If a dose is missed, the dose should be skipped, and the next dose is to be taken at the regularly scheduled time1
Dose adjustments for special populations1
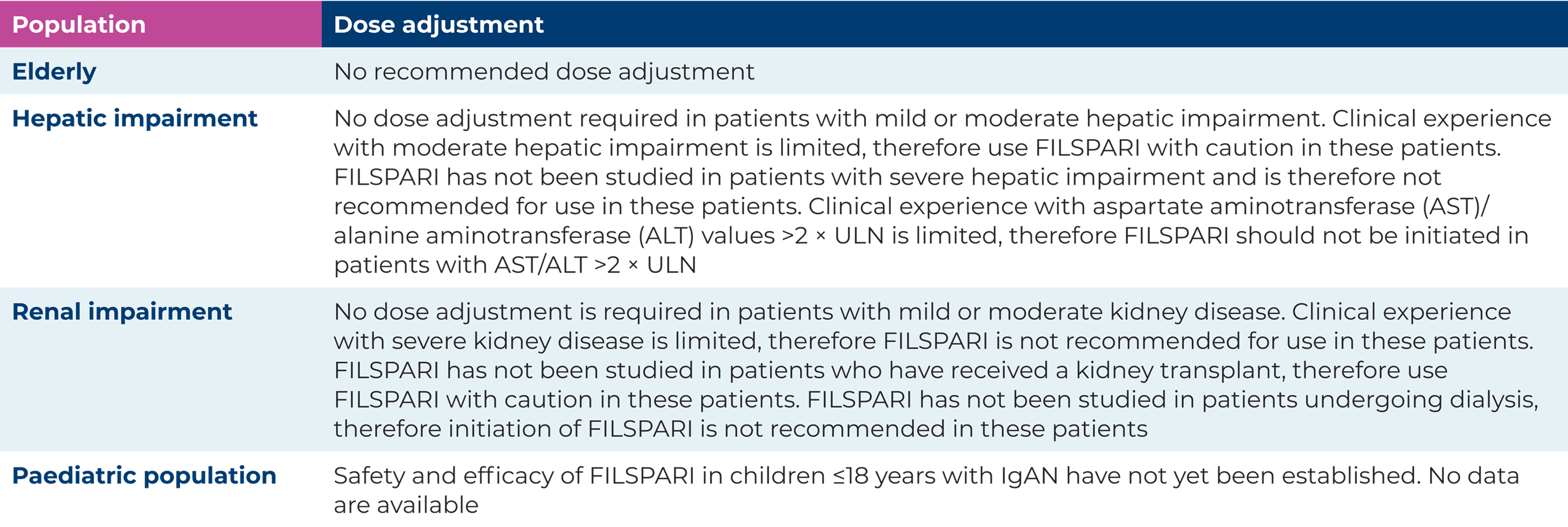

Special warnings and precautions for use1
Contraindications:
- Hypersensitivity to the active substance(s) or to any of the excipients
- Pregnancy
- Coadministration of angiotensin receptor blockers (ARBs), endothelin receptor antagonists (ERAs), or renin inhibitors
FILSPARI special warnings and precautions for use
Women of childbearing potential
FILSPARI treatment must only be initiated in women of childbearing potential when the absence
of pregnancy has been verified, and effective contraception is practiced. Exclude pregnancy before, during and for 1 month after treatment with FILSPARI has stopped. Women of childbearing potential have to use effective contraception during and up to 1 month after treatment has stopped. There are no or limited amount of data from the use of FILSPARI in pregnant women
Liver function
Elevations in ALT or AST of at least 3 × ULN have been observed with FILSPARI. No concurrent elevations in bilirubin >2 × ULN or cases of liver failure have been observed in FILSPARI treated patients. Therefore, to reduce the risk of potential serious hepatotoxicity, serum aminotransferase levels and total bilirubin
should be monitored prior to initiation of treatment and then continuously monitored every 3 months. Patients should be monitored for signs of hepatic injury. Avoid initiation of FILSPARI in patients with elevated aminotransferase (>2 × ULN) prior to drug initiation. Clinical experience with moderate hepatic impairment is limited, therefore FILSPARI should be used with caution in these patients
Breastfeeding
Physicochemical data suggest excretion of FILSPARI in human milk. A risk to newborns/infants cannot be excluded. FILSPARI should not be used during breastfeeding
Fertility
There are no data on the effects of FILSPARI on human fertility
Hypotension
Hypotension has been associated with the use of RAAS inhibitors, including FILSPARI. Hypotension may occur during treatment with FILSPARI and is reported more frequently in elderly patients. In
patients at risk for hypotension, eliminating or adjusting other antihypertensive medicinal products and maintaining appropriate volume status should be considered. If hypotension develops despite elimination or reduction of other antihypertensive medicinal products, dose reduction or dose interruption of FILSPARI should be considered. A transient hypotensive response is not a contraindication to further dosing of FILSPARI; treatment can be resumed once blood pressure has stabilised. FILSPARI should be used with caution in patients with systolic blood pressure values ≤100 mmHg. FILSPARI should not be uptitrated in patients with systolic blood pressure values ≤100 mmHg
Impaired kidney function
A transient increase in serum creatinine has been associated with RAAS inhibitors, including FILSPARI. A transient increase in serum creatinine may occur, especially when initiating treatment with FILSPARI. Periodic monitoring of serum creatinine and serum potassium levels should be performed in patients at risk. FILSPARI should be used with caution in patients with bilateral renal artery stenosis. Clinical experience in patients with an eGFR <30 mL/min/1.73 m2 is limited, therefore, FILSPARI is not recommended in these patients
Fluid retention
Fluid retention has been associated with medicinal products that antagonise the ETAR, including FILSPARI. Fluid retention may occur during treatment with FILSPARI. If fluid retention develops during treatment with FILSPARI, treatment with diuretics is recommended, or the dose of existing diuretics should be increased before modifying the dose of FILSPARI. Treatment with diuretics can be considered in patients with evidence of fluid retention before treatment initiation with FILSPARI. FILSPARI has not been studied in patients with heart failure, therefore, FILSPARI should be used with caution in patients with heart failure
Effects on ability to drive and use machines
FILSPARI may have minor influence on the ability to drive and use machines. No studies on the effects
of FILSPARI on the ability to drive and use machines have been performed. It should, however, be taken into account that dizziness may occur when taking FILSPARI. Patients with dizziness should be advised to refrain from driving or using machines until symptoms have subsided
Hyperkalaemia
Treatment should not be initiated in patients with serum potassium level >5.5 mmol/L. As with other medicinal products that affect the RAAS, hyperkalaemia may occur during treatment with FILSPARI,
especially in the presence of renal impairment and/or heart failure. Close monitoring of serum potassium in patients at risk is recommended. If patients experience clinically significant hyperkalaemia, adjustment of concomitant medicinal products, or temporary down-titration or discontinuation is recommended. If serum potassium level is >5.5 mmol/L discontinuation should be considered
Lactose
Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take this medicinal product
Sodium
This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’
Please consult the Summary of Product Characteristics for full safety information before prescribing
References & footnotes
Abbreviations
ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; AT1, angiotensin II type 1; BP, blood pressure; eGFR, estiamted glomerular filtration rate; ERA, endothelin receptor antagonist; ETA, endothelin type A; ETAR, endothelin type A receptor; IgA, immunoglobulin A; IgAN, immunoglobulin A nephropathy; K+, potassium; MoA, mechanism of action; RAAS, renin angiotensin aldosterone system; RASi, renin angiotensin system inhibitior; ULN, upper limit of normal
References
- FILSPARI SmPC.
- Rovin B, et al. Lancet. 2023;402(10417):2077–90.
- Barratt, J, et al. Front. Med. 2024;11:1461879.
- Campbell KN, et al. Int J Nephrol Renovasc Dis. 2023;16:281–91.
Additional
DE-FCM-2100195

FILSPARI is contraindicated during pregnancy. FILSPARI treatment must only be initiated in women of childbearing potential when the absence of pregnancy has been verified. Women of childbearing potential have to use effective contraception during and up to 1 month after treatment has stopped.
▼Adverse events should be reported. Reporting forms and information for the United Kingdom can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Vifor Pharma Ltd.
Tel: +44 1276 853633. E‑mail: MedicalInfo_UK@viforpharma.com
Please read the full SmPC prior to administration. FILSPARI® is a registered trademark.
UK-SPT-2500087 (v2.0) | Date of preparation: November 2025
Mechanism of Action
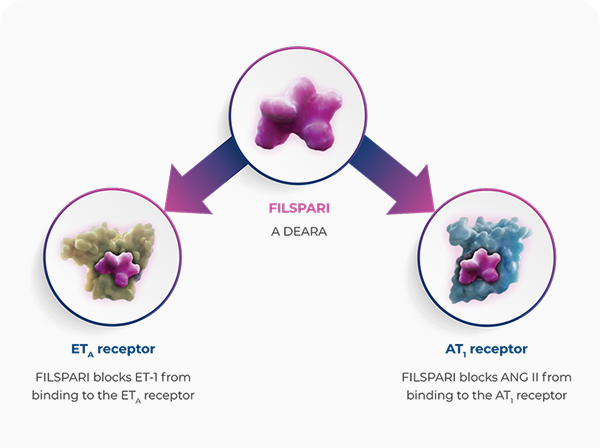
FILSPARI targets two critical pathways involved in IgAN disease progression in one molecule1,4,5
FILSPARI is a DEARA, a dual endothelin angiotensin receptor antagonist, and the first and only single molecule, non-immunosuppressive therapy indicated for patients with primary IgAN to reduce proteinuria1,4,5
FILSPARI targets two critical pathways, the endothelin and angiotensin pathways, involved in IgAN disease progression1,4,5
Endothelin 1, via ETAR, and angiotensin II, via AT1R, mediate processes that lead to IgAN progression through haemodynamic actions, mesangial cell proliferation, increased expression of pro-inflammatory and pro-fibrotic mediators, podocyte injury, and oxidative stress1
References & footnotes
Abbreviations
AT1, angiotensin II type 1; AT1R, angiotensin II type 1 receptor; DEARA, dual endothelin angiotensin receptor antagonist; ETA, endothelin type A; ETAR, endothelin type A receptor; IgA, immunoglobulin A; IgAN, immunoglobulin A nephropathy; MoA, mechanism of action
References
- FILSPARI SmPC.
- Rovin B, et al. Lancet. 2023;402(10417):2077–90.
- Barratt, J, et al. Front. Med. 2024;11:1461879.
- Campbell KN, et al. Int J Nephrol Renovasc Dis. 2023;16:281–91.
- Syed YY, et al. Drugs. 2023;83(6):563–8.
Additional
DE-FCM-2100195

FILSPARI is contraindicated during pregnancy. FILSPARI treatment must only be initiated in women of childbearing potential when the absence of pregnancy has been verified. Women of childbearing potential have to use effective contraception during and up to 1 month after treatment has stopped.
▼Adverse events should be reported. Reporting forms and information for the United Kingdom can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Vifor Pharma Ltd.
Tel: +44 1276 853633. E‑mail: MedicalInfo_UK@viforpharma.com
Please read the full SmPC prior to administration. FILSPARI® is a registered trademark.
UK-SPT-2500088 (v2.0) | Date of preparation: November 2025
Resources







References & footnotes
Abbreviations
IgAN, immunoglobulin A nephropathy; KDIGO, Kidney Disease: Improving Global Outcomes, UKKW, United Kingdom Kidney Week.
References
- FILSPARI SmPC.

FILSPARI is contraindicated during pregnancy. FILSPARI treatment must only be initiated in women of childbearing potential when the absence of pregnancy has been verified. Women of childbearing potential have to use effective contraception during and up to 1 month after treatment has stopped.
▼Adverse events should be reported. Reporting forms and information for the United Kingdom can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Vifor Pharma Ltd.
Tel: +44 1276 853633. E‑mail: MedicalInfo_UK@viforpharma.com
Please read the full SmPC prior to administration. Filspari® is a registered trademark.
UK-SPT-2500195 | Date of preparation: October 2025
Adverse events should be reported. Reporting forms and information for the United Kingdom can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Vifor Fresenius Medical Care Renal Pharma, care of Vifor Pharma Ltd.
Tel: +44 1276 853633. E-mail: MedicalInfo_UK@viforpharma.com.

Stay informed
Register with CSL Vifor for the latest releases. This will include promotional content
Register



